Most people know sodium thiosulfate as a basic industrial chemical. It has been used in large volumes for years, with little attention to purity.
That wasn’t a problem until doctors started finding new uses for it, like managing calciphylaxis or protecting patients from chemotherapy-related toxicity. Suddenly, the old industrial version wasn’t good enough anymore.
The purity was inconsistent, and the standard testing methods didn’t work because the compound breaks down the moment acid is added.
US12304813B2 addresses that gap by outlining how to produce high-purity, pharmaceutical-grade sodium thiosulfate and test it properly.
In fact, the patent’s assignee, Hope Medical, has already taken steps to protect this innovation, underscoring the centrality of this purification approach.
To understand where this patent sits in the wider purification landscape, we explored it using the Global Patent Search tool.
What US12304813B2 Is Really About
To understand this patent, it helps to start with the basic issue. We know sodium thiosulfate can support certain treatments, but the versions available today aren’t pure enough, and the usual testing methods don’t work because the compound breaks down during analysis. That is the exact gap US12304813B2 focuses on.
The inventors looked at why traditional sodium thiosulfate couldn’t meet modern medical standards. It carried tiny impurities, broke down during normal testing, and wasn’t made with pharmaceutical-grade consistency in mind. So the patent lays out two things very clearly,
how to clean the material properly, and how to test it without ruining the sample.
The process they describe removes metals, unwanted carbon, salts, and anything that could interfere with clinical use. Just as importantly, they introduce a testing method that finally works for this compound, something older techniques struggled with.
In simple terms, the patent turns sodium thiosulfate from an industrial ingredient into something reliable enough for sensitive medical treatments.
The Main Ideas Behind the US12304813B2
Let’s have a look at the core features the inventors focused on and the parts that actually make this patent work.
- Creating truly pharmaceutical-grade sodium thiosulfate: The patent lays out how to remove tiny impurities like metals, carbon residues, and carbonate so the final material meets modern medical standards.
- Fixing the testing problem: Traditional testing methods break down sodium thiosulfate or cause it to precipitate. This patent introduces a way to measure organic carbon accurately without damaging the sample.
- A purification process designed for consistency: From reacting the ingredients to filtering, concentrating, and crystallizing, the steps are designed to deliver the same clean quality every time.
- Making the compound usable for newer treatments: By improving purity and testing, the invention makes sodium thiosulfate suitable for expanded clinical uses, not just older grandfathered applications.
These elements highlight how the patent moves the compound toward higher purity, better testing, and real-world therapeutic use.
As more treatments start depending on cleaner inputs, the entire manufacturing chain tightens up. Even in cancer-therapy drug processing, similar shifts toward impurity-controlled chemistry have become essential.
Similar Patents That Help Complete the Picture
To understand where this patent fits in the bigger picture, it helps to look at the patents that came before it and shaped the same technical space.
Using the Global Patent Search tool, we traced older ideas that deal with purification, impurity control, and reliable testing of sensitive chemical compounds.
Each of these patents tackles a piece of the same problem from a different angle, and together they show how the field gradually moved toward the high-purity, pharmaceutical-grade approach described in US12304813B2.
Let’s explore some of them:
1. US4590058A
US4590058A is one of the earlier attempts to refine sulfur-based compounds at scale, a problem that has always been trickier than it sounds.
Filed by Olin Corporation in 1985, the patent focuses on improving the purity of alkali metal hydrosulfite solutions. These are the materials that routinely end up contaminated with thiosulfates, sulfites, and other leftovers from production.
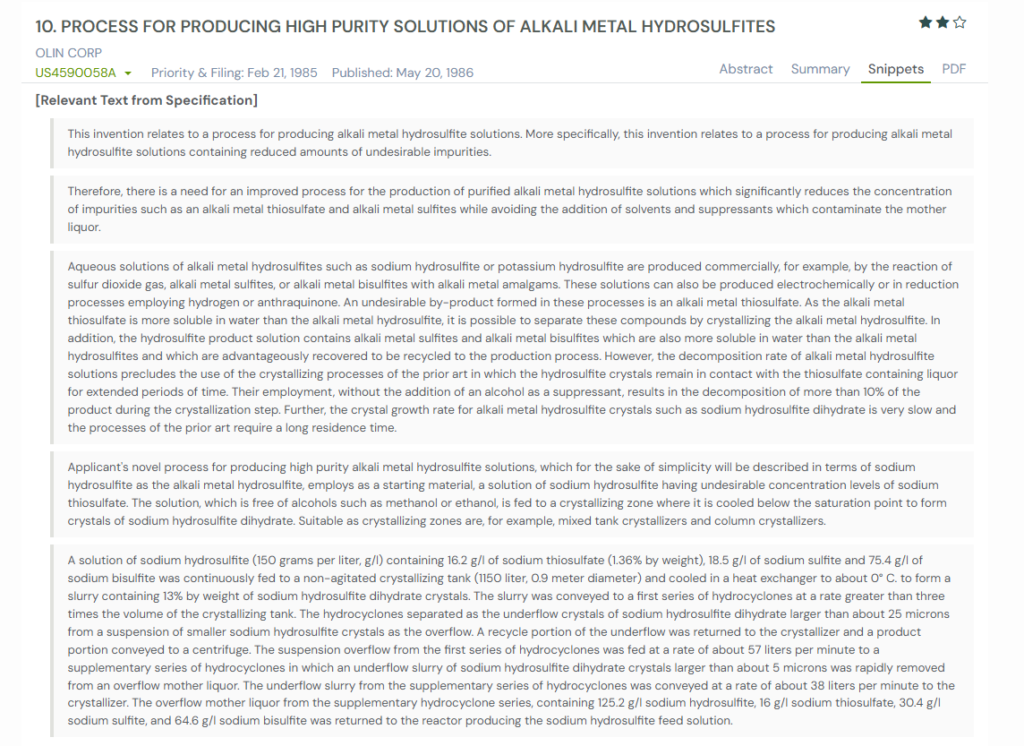
The engineers realised that older crystallization methods were too slow and unstable. The product decomposed if it sat too long, and adding alcohol suppressants only introduced new contaminants. So they designed a faster, tightly controlled crystallization loop that removes larger, cleaner crystals quickly while sending impurity-heavy liquid back into the system.
What makes this patent especially relevant to US12304813B2 is the mindset behind it. Both inventions tackle the same root issue. That is, how do you purify a sensitive sulfur compound without causing it to degrade or absorb impurities during the process.
Why This Patent Matters in the Bigger Picture
This early approach laid the groundwork for modern purification strategies by showing that sensitive sulfur compounds can be cleaned consistently without harsh additives.
This emphasis on batch-to-batch consistency reflects a broader trend in pharmaceutical ingredient design, highlighted in patents like US11589969B2, where stability and purity go hand in hand.
2. US4810483A
US4810483A tackles a problem that chemists have struggled with for years. The problem is how to turn unstable hydrate crystals of alkali metal hydrosulfites into a clean, stable anhydrous form without causing the material to fall apart.
Filed by Olin Corporation in 1987, the patent focuses on producing larger, purer anhydrous crystals by carefully controlling pH and temperature during the dehydration step.
Earlier industrial methods relied heavily on alcohol, which reduced solubility but also introduced problems such as contamination, slow separation, and faster crystal decomposition.
This invention avoided that by using an alkaline dehydration process that kept the pH high enough to protect the compound while converting it into a more stable form.

What makes this relevant to US12304813B2 is the shared challenge behind both patents. Both work with sulfur-based compounds that are sensitive, impurity-prone, and quick to degrade if handled the wrong way.
Why This Patent Matters in the Bigger Picture
By proving that fragile sulfur compounds can be stabilized and purified without harsh additives, US4810483A contributed to the foundation that modern high-purity processes build on. Its lessons in stability, controlled dehydration, and impurity reduction support the same goals behind today’s efforts to produce medically reliable sodium thiosulfate.
Even plant-derived formulations face similar purity hurdles, as seen in cannabis-based skincare patents like US10653736B2 where stabilizing sensitive compounds becomes central to product safety.
3. AU2022200542B2
AU2022200542B2 explores a very different challenge from the industrial purification processes we saw in earlier patents, but it still sits in the same medical landscape.
Filed in 2022 by ETH Zurich and the University of Bern, this patent explores methods to prevent calcium from crystallizing in the body. This kind of crystallization is common in chronic kidney disease and often leads to stiff arteries, kidney stones, and other serious complications.
The patent introduces modified inositol compounds that can slow or block the transition of early calciprotein particles into the harder crystalline forms that eventually drive tissue calcification.
By attaching solubility enhancing groups like PEG or polyglycerol, the inventors created molecules that stay active longer and interact with the calcium phosphate particles before they become harmful.
This connects back to our main sodium thiosulfate patent because both patents address problems rooted in abnormal mineral behavior. One focuses on purifying and preparing a compound for therapeutic use. The other focuses on interrupting the crystallization process itself.
Why This Patent Matters in the Bigger Picture
Pathological calcium crystallization has no widely accepted therapy.
AU2022200542B2 shows a promising direction by targeting the earliest stage of the calcification process, which adds valuable context to the broader therapeutic landscape around sodium thiosulfate.
If you’re interested in how other chemical compositions are advancing medical treatments, our analysis of US6630507B1 explores how cannabinoids function as neuroprotective agents.
4. US5098679A
US5098679A focuses on a specific challenge that keeps recurring in sulfur chemistry. When you make alkali metal hydrosulfite solutions, impurities such as aluminum tend to remain dissolved and later interfere with bleaching performance.
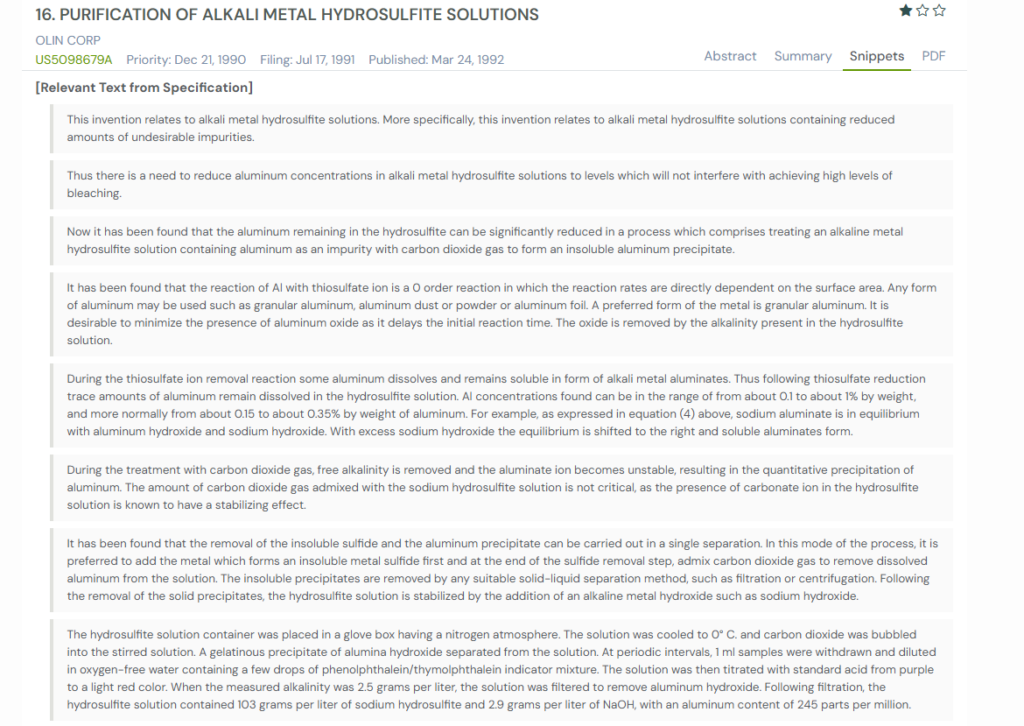
The patent, filed in 1991, tackles that problem head-on. It introduces a cleaner way to purify these solutions by removing both sulfide-based impurities and aluminum in one streamlined process.
At the heart of the patent is a smart trick. The inventors discovered that if you treat the solution with carbon dioxide gas, aluminum that normally stays dissolved becomes unstable and drops out as a solid precipitate.
Once that happens, the aluminum and other unwanted particles can be pulled out together through filtration or centrifugation. The end result is a hydrosulfite solution with dramatically lower impurity levels and much more predictable bleaching performance.
This idea connects back to our main patent because it highlights something the sodium thiosulfate world keeps running into. Contaminants like aluminum and sulfides do not just affect industrial processes.
They also complicate pharmaceutical-grade purification, stability, and regulatory compliance. US5098679A offers one more example of how the industry has been refining sulfur-based compounds to meet higher standards of quality and purity.
Why This Patent Matters in the Bigger Picture
By showing how to remove stubborn impurities at scale, US5098679A helps explain the long journey sulfur related chemistries have taken toward cleaner, more reliable formulations.
These earlier purification insights add context to the push behind modern filings like US12304813B2, where purity is not just a manufacturing goal but a requirement for safe clinical use.
A similar standard appears in EP4344633B1, where medical-grade wearables depend on components that remain stable and contamination-free under clinical conditions.
A comparable focus on formulation stability appears in US10869845B1, where precise pH control is used to keep ephedrine injectable solutions stable during long-term storage.
A Side by Side Look at the Similar Patents
Before we move ahead, it helps to look at all four related patents side by side. Each one solves a different problem, yet they all sit in the same broader space of improving purity, stability, or therapeutic value in sulfur based or crystallization related chemistries.
A quick comparison makes it easier to see how they complement the goals of our main patent.
| Patent Number | What the Patent Is About | Key Focus | Core Mechanism | Connection with US12304813B2 |
| US4590058A | Producing high purity alkali metal hydrosulfite solutions | Removing impurities like thiosulfate, sulfites, and bisulfites from hydrosulfite mixtures | Crystallizing hydrosulfite under controlled cooling and separating crystals through hydrocyclones to reduce contamination | Shows early chemical purification strategies that mirror the effort to control impurities in sodium thiosulfate for pharmaceutical use |
| US4810483A | Creating stable anhydrous hydrosulfite crystals with fewer impurities | Improving stability and purity of sulfur based compounds during crystallization | Converting hydrate crystals to anhydrous crystals using alkaline dehydration while maintaining pH to prevent decomposition | Highlights how careful control of pH, impurities, and crystal formation informs later pharmaceutical grade purification |
| US5098679A | Purifying hydrosulfite solutions by removing aluminum and sulfide impurities | Reducing metal contamination to improve bleaching and stability | Bubbling carbon dioxide into the hydrosulfite solution to precipitate aluminum, then removing solids through filtration | Demonstrates impurity removal techniques that align with the purity goals and metal limits required for sodium thiosulfate in the main patent |
| AU2022200542B2 | Modified inositol compounds that prevent harmful calcium crystallization in the body | Stopping progression of early calciprotein particles into harder crystalline forms | Using inositol derivatives with solubility enhancing groups like PEG to interact with calcium phosphate particles | Adds medical context by showing how controlling crystallization is central to treating conditions related to calcium imbalance, similar to therapeutic uses of sodium thiosulfate |
Seeing the Broader Landscape with the Global Patent Search Tool
Chemical purification doesn’t move in a straight line. Every patent adds one small improvement. One fixes impurities, one improves crystal stability, and another solves a testing challenge. You only see the full picture when they’re placed next to each other, and that’s exactly what the Global Patent Search tool helps you do.
The Global Patent Search tool maps how earlier hydrosulfite and thiosulfate technologies evolved and how they led to the pharmaceutical-grade expectations behind US12304813B2.
Here’s how GPS simplifies that discovery:
1. Start Anywhere: Search for US12304813B2 or even describe the idea. GPS immediately surfaces patents on purification, impurity removal, crystallization control, or analytical testing.
2. Spot the Overlaps: GPS highlights short claim snippets showing how earlier patents handled thiosulfate removal, carbonate limits, or aluminum precipitation. You can quickly see where the ideas align.
3. Open the Details When Needed: Click into any patent to explore reaction steps, filtration methods, crystallization paths, or stability issues that shaped later pharmaceutical-grade processes.
4. Find Unexpected Connections: GPS often surfaces patents from paper bleaching, textile chemistry, or industrial hydrosulfite production. Many faced the same impurity and stability challenges that modern sodium thiosulfate must overcome.
By turning scattered references into a connected view, GPS helps you understand where US12304813B2 fits in the broader evolution of sodium thiosulfate purification and medical-grade quality standards.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The similar patent references mentioned are preliminary results from the Global Patent Search tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.
Frequently Asked Questions
1. What makes sodium thiosulfate difficult to purify?
Sodium thiosulfateis harder to purify because it breaks down easily when exposed to acids. The moment it touches acid, it starts breaking down and forms sulfur and salt particles. These tiny bits block filters, mess with equipment, and make standard impurity tests fail. So even a routine purification step turns into a complicated process.
2. Why can’t regular NPOC testing be used for sodium thiosulfate?
The typical NPOC test starts by adding acid to remove inorganic carbon. But sodium thiosulfate degrades the moment acid is added, creating sulfur and salts that interfere with the analysis. This is why a new, specialized testing method is needed.
3. Why does pharmaceutical-grade sodium thiosulfate need such strict purity levels?
Sodium thiosulfate needs strict purity levels because it’s used in treatments where even small impurities can cause problems. When a compound is going into patients for things like cyanide poisoning or chemotherapy support, the material has to be clean, stable, and predictable. Tiny amounts of metals, organic carbon, or carbonate can affect how the treatment works and how safe it is, so the purity bar has to be much higher than the industrial version.