Norepinephrine is one of the most critical drugs in emergency care, used to stabilize dangerously low blood pressure. Yet in liquid form, it breaks down quickly, leaving hospitals struggling with short shelf lives and frequent replacements. Past attempts to address this issue often relied on antioxidants, but these carry their own safety risks.
Patent US12245996B2, currently at the center of a lawsuit between Nevakar Injectables and Baxter Healthcare, takes a different path. By controlling pH, adding a salt, and using a chelating agent, it creates a ready-to-inject norepinephrine solution that remains stable without antioxidants. This makes it safer for patients, easier for hospitals, and more reliable in urgent situations.
To see how this invention fits into the broader effort to make emergency medicines more durable, we turned to the Global Patent Search (GPS) tool. The tool identified similar patents on norepinephrine, epinephrine, and other catecholamines, all exploring different methods to extend shelf life and ensure these life-saving drugs are readily available when needed.
Let’s take a look at what GPS found, albeit after a quick look at the key features of the subject patent.
Breaking Down Patent US12245996B2
US12245996B2 focuses on creating a ready-to-inject norepinephrine composition that remains stable over time without using antioxidants. Norepinephrine is a critical drug used to treat low blood pressure and cardiac emergencies, but it degrades quickly, especially in liquid form.
This patent solves that problem by designing a liquid formulation that maintains its strength and purity for months, even at higher temperatures. The invention also allows for heat sterilization, making it safe for clinical use right out of the package.
The Key Features Of This Patent Are
1. Antioxidant-Free Formula
The composition avoids common antioxidants like sodium metabisulfite, reducing risk of allergic reactions.
2. Stable R-Isomer Content
At least 90% of the norepinephrine remains in its active R-isomer form after 3 months.
3. Low Degradation Rate
Less than 5% of the total norepinephrine breaks down over a 3-month storage period.
4. Controlled pH Range (3.7–5.0)
A specific acidic pH range improves stability and reduces chemical breakdown.
5. Chelating Agent (1–100 μg/mL)
A bicarboxylic acid chelator helps prevent oxidation by binding metal ions.
6. Pharmaceutically Acceptable Salt (e.g., NaCl)
Salt levels between 0.6% and 1.2% maintain tonicity for safe injection.
7. Ready-to-Use Liquid Form
The drug is pre-diluted and sterile, removing the need for mixing before use.
8. Suitable for Autoclaving
The solution can be heat sterilized without significant degradation or isomerization.
9. Low Oxygen Packaging
Use of deoxygenated water and oxygen scavengers helps prevent oxidation during storage.
10. Multiple Dose Options
Available in different concentrations: 16, 32, and 64 μg/ml, to meet varying clinical needs.
This patent addresses a critical challenge in drug formulation, stabilizing a delicate, essential medication. By eliminating antioxidants and improving shelf life, US12245996B2 offers a safer, more convenient option for emergency care settings.
Related Read: See how EP3536712B1 and similar patents focus on thermal resilience in injectable drug formulations, just like US12245996B2 does for stabilizing norepinephrine without antioxidants.
Similar Patents To US12245996B2
To explore the formulation strategies behind US12245996B2, we used the Global Patent Search tool to find similar patents. These references also aim to improve the stability and safety of injectable norepinephrine. While each follows a different formulation path, they all focus on extending shelf life, avoiding reconstitution, and reducing degradation.
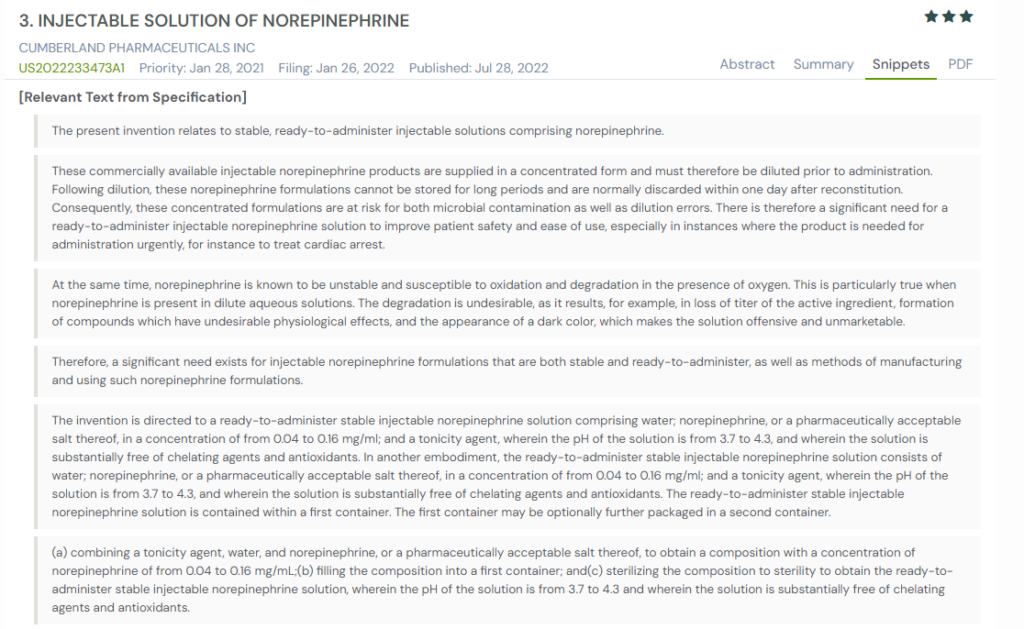
1. US2022233473A1
This U.S. patent, US2022233473A1, published in July 2022, describes a ready-to-administer injectable norepinephrine solution that does not need to be diluted before use. It targets the common problems seen in existing products: short shelf life, instability after reconstitution, and risks of contamination. The goal is to deliver a stable product that can be stored at room temperature and used directly from its container in emergency settings.
Below, we have added snapshots from the GPS tool displaying the relevant sections from similar patents.
What This Patent Introduces To The Landscape
- A liquid norepinephrine formulation with concentrations from 0.04 to 0.16 mg/mL
- No added antioxidants or chelating agents, formulated to be “substantially free” of both
- Controlled pH between 3.7 and 4.3 for better chemical stability
- Long-term stability demonstrated at both 25°C and 40°C
- High retention of active L-isomer (≥90%) over 6 to 24 months
- Reduced oxygen exposure using nitrogen sparging (≤1 ppm dissolved oxygen)
- Packaged in either glass vials or IV bags, suitable for direct IV injection
- Can be sterilized via autoclaving after final packaging
- Avoids reconstitution or dilution before administration
- Meets ICH and USP impurity and assay guidelines during storage
How It Connects To US12245996B2
- Both are ready-to-inject norepinephrine solutions designed for long-term stability
- Each uses a pH range of 3.7–4.3 to reduce degradation
- US12245996B2 includes a chelating agent; this patent excludes it entirely
- Both minimize oxidation but use different strategies: oxygen scavenging vs. nitrogen sparging
- Each formulation is sterilized by autoclaving to ensure safety without harming the drug
Why This Matters
US2022233473A1 offers a simplified yet effective approach to norepinephrine stability. By removing extra stabilizers and relying on packaging and pH control, it presents an alternative path to achieving long shelf life. It addresses the same problem tackled by US12245996B2, but through a more minimal formulation strategy.
You can also see this theme reflected in US12304813B2, where the purification workflow is redesigned to deliver a stable, pharmaceutical-grade form of sodium thiosulfate.
2. WO1994013274A1
This international patent application, WO1994013274A1, published in June 1994, focuses on stabilizing catecholamine solutions, such as dobutamine, without using sulfite-based antioxidants. It addresses the risks linked to sulfite allergies by offering an alternative that uses metal ion chelators and pH control to prevent oxidation.
The solution remains stable even in the presence of oxygen and does not require cold storage, making it suitable for long-term use in clinical settings.
What This Patent Introduces To The Landscape
- A stabilized catecholamine solution using a metal ion chelator and acidic pH control
- Avoids the use of sodium bisulfite or metabisulfite as antioxidants
- Preferred pH range of 1.5 to 4.0, with 2.5 being most effective
- Use of EDTA or similar chelators (EGTA, DTPA, nitrilotriacetic acid)
- Chelator concentration from 0.01 to 1 mg/mL, in stoichiometric excess
- Demonstrated protection from oxidation without special storage
- Stable even with atmospheric oxygen exposure
- Can be used to increase cardiac contractility in emergency care
- pH adjusted using standard acids or bases (e.g., HCl or NaOH)
- Shelf life extended to three months without degradation or color change.
How It Connects To US12245996B2
- Both use metal ion chelators to prevent oxidative degradation.
- Each relies on acidic pH to support drug stability
- US12245996B2 targets norepinephrine; this patent focuses on dobutamine, a related catecholamine
- Both avoid traditional antioxidants like sulfites due to allergy risks
- Each demonstrates stability without refrigeration, useful in emergency settings
Why This Matters
WO1994013274A1 introduced a sulfite-free stabilization strategy for injectable catecholamines over 30 years ago. It helped set the stage for modern formulations like US12245996B2, which builds on these ideas for norepinephrine. They are using similar pH and chelation techniques while improving storage conditions and drug purity.
3. CA3093725A1
This Canadian patent, CA3093725A1, published in September 2019, describes a ready-to-administer epinephrine formulation with long-term stability. It addresses the instability of diluted epinephrine, which quickly degrades in the presence of light, air, or heat.
The patent outlines methods to extend shelf life without relying on antioxidants by adjusting pH, minimizing oxygen exposure, and incorporating a chelating agent. The formulation is designed for direct injection and can be sterilized through autoclaving without degrading the drug or increasing harmful isomers.

What This Patent Introduces To The Landscape
- A ready-to-administer epinephrine solution with a pH between 3.0 and 4.7
- Compositions that are antioxidant-free and stable for extended periods
- Use of metal ion chelators (e.g., EDTA, EGTA, DTPA) at low concentrations
- Minimization of dissolved oxygen to less than 1.5 ppm
- Packaging in polymer bags under inert gas with oxygen scavengers
- Terminal sterilization using autoclaving at 121°C for up to 15 minutes
- Maintains over 98% of active R-isomer, even after months of storage
- Total impurities remain under 0.7% and S-isomer content under 2%
- Formulated at very low epinephrine concentrations (e.g., 0.005–0.07 mg/mL)
- Compatible with flexible IV bags, BFS, and aluminum overwrap packaging
How It Connects To US12245996B2
- Both are antioxidant-free, sterile, and ready-to-administer injectable solutions
- Each targets oxygen-sensitive drugs prone to degradation in aqueous forms
- Both use acidic pH ranges and chelating agents for stability
- Designed to avoid reconstitution and reduce medication errors
- Compatible with terminal sterilization without increasing isomer content
Why This Matters
This Canadian filing reveals how low-pH, chelator-based formulations improve epinephrine stability without antioxidants. Its strategies mirror those in US12245996B2, reinforcing best practices for long-term storage, safety, and simplified administration of injectable medications.
Similar stability challenges in emergency injectables are addressed in US10869845B1, which shows how ephedrine can be formulated as a ready-to-use solution that remains stable for months.
4. US2023140033A1
This U.S. patent application, US2023140033A1, published in May 2023, describes injectable epinephrine formulations designed for long-term stability. The formulation maintains over 94.5% API retention even after 30 months at room temperature.
The inventors focused on reducing degradation, discoloration, and overage: challenges common in commercial epinephrine products. This results in a formulation that is stable, safe, and effective at low epinephrine concentrations without needing refrigeration or excess antioxidant load.
What This Patent Introduces To The Landscape
- Epinephrine formulations with API recovery above 94.5% after 30 months at 25°C
- Use of EDTA at 1–8 μg/mL, avoiding zinc depletion or injection-site discomfort
- Low sodium metabisulfite levels (0.04–0.08 mg/mL) to reduce oxidation
- pH control between 3.6 and 4.0 using citric acid and sodium citrate
- Protection via nitrogen atmosphere filling and low headspace oxygen
- No API overage, avoiding unnecessary high dosages and side effects
- Buffers designed to maintain pH stability over time
- Formulations free of tartrate to reduce degradation risks
- Shown to resist discoloration and impurity buildup in high-heat storage tests
- Suitable for use in glass vials and pre-filled syringes
How It Connects To US12245996B2
- Both aim for long-term stability of oxygen-sensitive injectables
- Each uses low concentrations of EDTA as a stabilizer
- Maintain an acidic pH range for optimal degradation resistance
- Designed to avoid overage and reduce impurity formation
- Use inert gas filling and robust packaging to minimize oxidation
Why This Matters
This patent shows how small tweaks, like reduced EDTA and antioxidant use, can lead to stable, injectable epinephrine products with long shelf life. Its stability strategy supports goals shared with US12245996B2, especially in minimizing degradation without compromising safety or effectiveness.
Related Read: See how US12122789B2 strengthens drug stability through solid forms, echoing the same push for longer shelf life found in norepinephrine formulation patents.
5. CN106061467B
This Chinese patent, CN106061467B, published in February 2021, describes a method to produce low-concentration norepinephrine injectable solutions that remain stable without using antioxidants or preservatives.
The method allows terminal sterilization at 121°C, which is usually difficult for sensitive compounds like norepinephrine, without compromising the active ingredient. The resulting solution stays chemically stable at room temperature for at least 6 months.

What This Patent Introduces To The Landscape
- Injectable norepinephrine solutions at 0.04–0.2 mg/mL concentration
- No use of antioxidants, sulfites, or preservatives
- Precise pH control (3.2–3.6) to prevent degradation and racemization
- Oxygen-free processing using nitrogen purging and inert conditions
- Terminal sterilization possible at 121°C for 15 minutes
- Stability maintained at 25°C for 6 months and 40°C for 3 months
- Impurity levels kept below 0.05% (e.g., arterenone and d-norepinephrine)
- Compatible with pharmaceutically acceptable salts like bitartrate
- Packaged in glass vials under nitrogen to prevent oxidation
- Enables safer and simpler manufacturing without allergen risks
How It Connects To US12245996B2
- Both address stability challenges in low-dose catecholamine formulations
- Each avoids sulfites and harsh preservatives
- Prioritize low oxygen environments and careful pH control
- Enable terminal sterilization without degrading the active ingredient
- Aim to extend shelf life at room temperature without cold chain reliance
Why This Matters
This patent shows how norepinephrine, like epinephrine, can be stabilized at low concentrations without needing antioxidants. It highlights the growing trend of preservative-free, ready-to-use injectables, offering safer options for patients with sensitivities while simplifying hospital workflows.
Related Read: See how US10828310B2 tackles clot-related emergencies by combining targeted anticoagulants and antiplatelets, a dual-action approach also driving biotech drug pipelines.
How to Find Related Patents Using Global Patent Search
Understanding the landscape around injectable epinephrine formulations is key when developing stable, low-dose, and preservative-free solutions. The Global Patent Search tool helps identify similar patents that tackle similar formulation and stability issues.
Here’s how you use the GPS tool:
- Enter the patent number: Start by entering a number like US12245996B2 into GPS tool. Use keywords like “low pH,” “EDTA,” or “oxidation-stable” to narrow your search.
- Review curated snippets: GPS now shows highlighted text instead of full claims. These snippets focus on solutions for stability, impurity control, and extending shelf life.
- Find related technologies: The tool reveals other patents working on similar problems, like antioxidant-free systems, terminal sterilization, or metal ion management.
- Compare technical strategies: Rather than matching claims, GPS shows how others solve challenges with pH buffering, inert gas packaging, or low-dose injection design.
- Connect across domains: GPS helps link ideas across areas like emergency care, IV drug delivery, and pre-filled syringes, offering broader insight into formulation trends.
Want to boost your patent coverage quickly? Here are practical tips on how to conduct a patent search faster.
Whether you are working on new drug formulations or improving delivery systems, Global Patent Search helps you uncover technical insights and stay ahead of emerging trends.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.