Making a medicine work isn’t only about finding the right molecule. The same focus on chemical and packaging choices appears in US12245996B2, which shows how pH control and chelation can extend norepinephrine’s shelf life without antioxidants. It’s also about shaping it into the right solid form. The manner in which a drug crystallizes can impact its stability, shelf life, and ease of manufacture. For rare disease treatments, where reliability matters most, this detail can make all the difference.
That’s the focus of US12122789B2, a patent on risdiplam, a drug used to treat spinal muscular atrophy (SMA). It introduces two solid forms: Form A, a dry crystal that resists moisture, and Form D, a hydrate that contains water. Together, these versions make the drug more stable, easier to produce, and safer to deliver.
The patent is part of the litigation suit between Genentech, Inc. et al. v. Zydus Lifesciences Global FZE et al., but our interest here is the science.
To see how this invention fits into the bigger effort to make medicines more dependable, we used the Global Patent Search (GPS) tool. The tool surfaces similar patents on solid forms, revealing how researchers worldwide are tackling the same challenge of drug stability and scalability to make medicines stronger and more dependable.
Understanding Patent US12122789B2
Patent US12122789B2 focuses on two special solid forms of a medicine called risdiplam, a treatment for spinal muscular atrophy. These solid versions, known as Form A and Form D, offer better control during manufacturing and enhance the storage and delivery of the medicine.
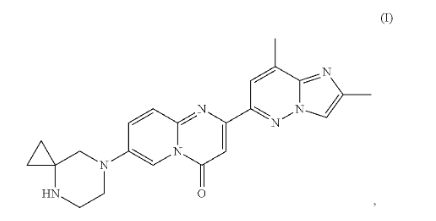
Source: Google Patents
The Key Features Of This Patent Are
1. Form A is a dry, crystal form that does not easily absorb water from the air.
2. Form D includes water in its structure, forming what’s known as a crystalline hydrate.
3. Form A is more chemically stable, which helps keep the drug effective for longer.
4. It filters easily during production, helping reduce waste and speed up the process.
5. Form A can be identified by distinct peaks in its XRPD (X-ray powder diffraction) patterns.
6. The invention also covers other solid types, such as Forms B, C, E, F, and G, though less emphasis is placed on them.
7. Raman and IR spectroscopy are used to confirm the identity of each form.
8. The patent also provides exact steps to create both Form A and Form D using solvents, temperature control, and seeding methods.
9. Salt versions of risdiplam are included, such as hydrochloric, tartaric, and citric acid forms.
10. Each form reaches high purity levels, making them safer and more reliable in medicine.
US12122789B2 highlights how small changes in solid form can improve a drug’s real-world performance. These innovations support safer, faster, and more consistent medicine production.
Did you know this mirrors trends highlighted in extended-action peptide drug patents, which also explore how formulation changes improve safety and effectiveness.
Similar Patents To US12122789B2
Building on the risdiplam patent, we used the Global Patent Search (GPS) tool to identify related inventions that tackle the same issue: how to make solid drug forms more stable, scalable, and dependable. These patents cover everything from crystal form discovery to testing methods, each offering a different path toward safer and more effective medicines.
1. WO2015123801A1
This international patent, WO2015123801A1, published in August 2015, focuses on methods to prepare different crystal forms (polymorphs) of a drug compound called 6-(4-chlorophenoxy)-tetrazolo[5,1-a]phthalazine. It explains how changes in crystal structure can impact important properties such as solubility, stability, and drug performance.
The patent also outlines practical steps for discovering and testing these crystal forms to find the best version for use in medicine.
Below, we have added snapshots from the GPS tool displaying the relevant overlap from similar patents.

What This Patent Introduces To The Landscape
- Explains how one compound can exist in many different crystal forms
- Shows that each form can affect drug behavior, such as solubility, flow, and melting point
- Highlights how crystal type influences drug safety, storage, and effectiveness
- Uses crystallization techniques like cooling, evaporation, and suspension to find new forms
- Describes high-throughput systems to run hundreds of crystal experiments quickly
- Applies solid testing tools like XRPD, Raman, IR, and DSC to study crystal properties
- Compares stability and water absorption between crystal types
- Offers a step-by-step process for turning one form into another in a solution
- Focuses on selecting the most stable and process-friendly form for production
- Supports better manufacturing by choosing crystal types that are easier to filter and store
How It Connects To US12122789B2
- Both patents focus on developing and optimizing solid crystal forms of active pharmaceutical ingredients.
- Each highlights specific forms (Form A, Form B, Form D) with unique characteristics.
- Both use XRPD and other tests to confirm and study these crystal structures.
- Each patent includes methods to prepare and select the most stable form.
- Both aim to improve drug safety, storage, and manufacturability by controlling crystal form.
- Each highlights the importance of choosing the right form during drug development.
Why This Matters
WO2015123801A1 shows that choosing the right crystal form can make a drug safer and more reliable. Like US12122789B2, it helps manufacturers control drug quality, stability, and performance from lab to patient.
Related Read: See how US10828310B2 tackles cardiovascular risk reduction with rivaroxaban plus aspirin, backed by large-scale clinical trial evidence shaping modern treatment strategies.
2. US2019106402A1
This U.S. patent application, US2019106402A1, published in April 2019, focuses on the physical form of a selective glucocorticoid receptor modulator (SGRM). It describes the development of a crystalline form, known as Form C, which offers better chemical and physical stability for pharmaceutical use.
The invention also discusses how crystallinity levels affect how well the drug can be stored, handled, and processed during manufacturing.

What This Patent Introduces To The Landscape
- Highlights the need for improved SGRM compounds with better potency, stability, and selectivity
- Emphasizes the role of crystallinity in improving shelf-life and drug performance
- Introduces Form C, a solid form identified by specific XRPD (X-ray powder diffraction) peak patterns
- Offers a range of crystallinity (from 50% up to 100%) depending on the form and conditions
- Uses Differential Scanning Calorimetry (DSC) to measure melting points of the crystalline form
- Describes controlled crystallization using ethanol-water solutions and temperature-based seeding
- Explains how variations in equipment and sample can slightly shift XRPD peak readings
- Provides detailed methods for purifying and isolating Form C on a large scale
- Includes conditions for drying, seeding, cooling, and crystallization to control solid formation
- Targets improved drug manufacturing through consistent solid-state properties.
How It Connects To US12122789B2
- Both patents describe the creation and selection of specific crystalline drug forms.
- Each provides XRPD peak data to identify the solid forms uniquely.
- Both emphasize stability and processability as key benefits of solid-state control.
- Each patent includes precise methods for crystallization and isolation of pure forms.
- Both focus on scaling up drug production while maintaining a consistent solid form.
- Each invention supports better shelf life and reliable drug performance through form selection.
Why This Matters
US2019106402A1 adds to the growing focus on solid drug forms that are easier to make and store. Like US12122789B2, it proves that controlling crystallinity is essential for building safer and more effective medicines.
Related Read: See how US12128039B2 refines quinoline-based cancer drugs with cleaner synthesis and stable tablet forms, addressing the same reliability challenges tackled by solid-form drug patents.
3. US2009270442A1
This U.S. patent application, US2009270442A1, published in October 2009, focuses on a wide range of polymorphic forms of a hydrochloride salt of a kinase-inhibiting compound (referred to as Compound 1).
The patent outlines multiple crystalline and amorphous forms, each with distinct physical characteristics like X-ray diffraction patterns and thermal behaviors. These forms are developed to optimize how the drug performs, is manufactured, and is delivered to patients.
What This Patent Introduces To The Landscape
- Identifies at least fifteen distinct crystalline forms (Forms A–P) and one amorphous form of Compound 1
- Provides X-ray powder diffraction (XRPD) data for each form to identify them uniquely
- Describes differential scanning calorimetry (DSC) results showing the melting and thermal behaviors of each form
- Introduces hydrate and solvate forms, including monohydrates, channel hydrates, and solvates with solvents like DMA, DMF, NMP, and THF
- Highlights stable and metastable forms, and their ability to convert between one another under certain conditions (e.g., humidity, temperature)
- Offers specific methods for producing each form using solvent systems, temperature control, and anti-solvent addition
- Emphasizes the importance of crystallinity and water content in determining stability and form selection
- Details form interconversion studies, including how some forms revert to more stable ones such as Form A under ambient or humid conditions.
- Includes formulation options for oral, topical, and injectable dosage forms based on the polymorphic form used
- Suggests dosage ranges and therapeutic uses in kinase-related diseases such as Parkinson’s disease, ALS, and cognitive disorders
- Describes how certain forms are more thermodynamically favorable and easier to scale in manufacturing
- Demonstrates how solid-state form impacts drug solubility, stability, and performance over time
How It Connects To US12122789B2
- Both patents emphasize identifying and characterizing multiple polymorphs of an active pharmaceutical ingredient.
- Each uses XRPD and DSC to define the unique signatures of different solid forms.
- Both include methods for the scalable production of specific crystalline or amorphous forms.
- Each focuses on form stability under different environmental conditions, such as humidity or heat.
- Both aim to improve drug formulation, storage, and performance by selecting the most suitable solid-state form.
- Each patent addresses therapeutic use cases where maintaining solid-state consistency is vital for clinical reliability.
Why This Matters
US2009270442A1 presents a comprehensive approach to solid-state drug design by covering a wide spectrum of crystal and amorphous forms for a kinase inhibitor. Like US12122789 B2, it demonstrates that polymorphism ensures drugs are safe, effective, and manufacturable at scale.
This type of detailed polymorph screening enables pharmaceutical developers to select the most suitable form for therapeutic delivery.
Related Read: See how patents like US12245996B2 extend norepinephrine shelf life without antioxidants, echoing the same focus on stability and purity that drives solid-form drug innovations.
4. US2010056585A1
This U.S. patent application, US2010056585A1, published in March 2010, focuses on polymorph B of a pharmaceutical compound known as MS-275. MS-275 belongs to a class of compounds being studied for their potential in treating certain types of cancer and other serious conditions.
The patent outlines different solid forms of MS-275, including three polymorphs (A, B, C) and an amorphous phase, and provides detailed information on how to identify and use each form.

What This Patent Introduces To The Landscape
- Describes three distinct crystalline forms (Polymorphs A, B, and C) and an amorphous version of MS-275
- Provides detailed melting point data, with Polymorph B melting between 159–160°C
- Uses X-ray powder diffraction (XRPD) to distinguish each polymorph based on its unique diffraction patterns
- Applies FT-Raman spectroscopy as an additional method to identify and differentiate the solid forms
- Notes that the amorphous form lacks sharp XRPD peaks, making it visually distinct from crystalline types
- Highlights manufacturing challenges, especially in reliably producing Polymorph A with high purity
- Presents Polymorph B as a stable and preferred form for pharmaceutical use
- Explains that all polymorphs can be used in oral and injectable drug formulations
- Describes multiple administration routes, including oral, intravenous, intramuscular, subcutaneous, and even implantation
- Mentions that Polymorph B can be combined with pharmaceutically acceptable carriers or diluents
- Notes that the dosage may vary depending on patient-specific factors like age, weight, or condition being treated
- Points to therapeutic applications, particularly in human patients and other warm-blooded animals
How It Connects To US12122789B2
- Both patents identify and characterize multiple polymorphs of an active pharmaceutical compound
- Each relies on XRPD and thermal analysis to clearly define the structural differences between forms.
- Both highlight the importance of form selection in drug development and formulation.
- Each emphasizes the practical challenges in the consistent manufacturing of specific forms
- Both recognize the impact of polymorphism on bioavailability, stability, and the administration route.
- Each explores flexible pharmaceutical applications, making the forms usable in a range of dosage types (oral, injectable, implantable)
Why This Matters
This patent reinforces the importance of choosing the right solid form of a drug early in development. Polymorph B of MS-275-like forms described in US12122789B2 offers a balance of stability, purity, and processability, making it suitable for real-world pharmaceutical applications. By using multiple analytical tools, the patent provides a clear pathway from lab discovery to safe and effective treatment.
5. MX2008015044A
This Mexican patent, MX2008015044A, published in December 2008, focuses on crystalline polymorphs of a compound used in pharmaceutical treatments. The compound, (R)-5-(2-aminoethyl)-1-(6,8-difluorochroman-3-yl)-1,3-dihydroimidazole-2-thione hydrochloride, can exist in multiple solid forms. The patent outlines how to prepare, purify, and analyze these forms, including Forms A, B, C, and X. It also discloses an amorphous version to improve drug quality and performance.
What This Patent Introduces To The Landscape
- Describes multiple polymorphs of the active compound: Forms A, B, C, X, and an amorphous form
- Uses aqueous HCl crystallization to produce Form A directly or through in-situ processes
- Shows that Form X can convert to Form B, which can later revert to Form A after drying
- Identifies Form C as the most thermodynamically stable polymorph based on interconversion studies
- Highlights high purity levels across crystalline forms, typically exceeding 97% by HPLC
- Details how solvent choice, humidity, and drying conditions affect which form is produced
- Uses XRPD to distinguish all forms, along with FT-Raman and Karl-Fischer titration methods
- Demonstrates polymorph stability tests under varying humidity and temperature over weeks
- Shows the amorphous form crystallizing into other polymorphs under certain solvent stress conditions
- Includes detailed experimental protocols for precipitation, evaporation, seeding, and vapor stress
- Provides yield percentages and melting points to help assess manufacturability and quality
- Supports pharmaceutical development by offering stable forms ideal for drug formulations
How It Connects To US12122789B2
- Both patents focus on the development and control of multiple polymorphic forms for pharmaceutical use.
- Each provides methods for crystallization and form interconversion using solvents, temperature, and humidity.
- Both apply XRPD and other analytical tools to confirm the identity and stability of solid forms.
- Each emphasizes form stability under humidity and heat, critical for long-term storage and handling.
- Both aim to produce thermodynamically favorable forms suitable for pharmaceutical manufacturing.
- Each patent supports the idea that form choice impacts purity, processability, and therapeutic value.
Why This Matters
MX2008015044A shows how fine control over crystal forms leads to better drug quality and stability. Like US12122789B2, it emphasizes how solid-state engineering can solve key challenges in drug production. These polymorphs are not just scientific curiosities; they are vital to delivering safe, stable, and effective medications.
Similar approaches to solid-state drug innovation can also be seen in US12144810B1 and similar pharmaceutical patents, which detail strategies for developing stable and scalable drug forms.
How To Find Similar Patents Using Global Patent Search
When developing new pharmaceutical compounds in solid form, it is critical to explore how other inventors have addressed similar challenges. The Global Patent Search tool is designed to help identify related patents that focus on polymorph development, crystallization processes, and solid-state characterization.
This approach not only supports better research and development but also helps reduce overlap with existing technologies. Here’s how to use the GPS tool to find patents related to US12122789B2:
- Search by patent number or keywords: Begin by entering US12122789B2 into the Global Patent Search tool. You can also search with terms like “crystalline drug form,” “XRPD profile,” or “polymorph preparation.”
- Review key snippets: Instead of long documents, GPS now shows short, curated text excerpts. These focus on important elements like stability, purity, and crystallization techniques, making it easier to evaluate relevance at a glance.
- Identify similar patents: GPS presents a list of patents that address the same technical goals, such as improving manufacturability, purity, or shelf stability through polymorph selection.
- Compare crystallization strategies: The tool shows how different researchers tackle solid form development using techniques like solution cooling, solvent evaporation, or in-situ crystallization.
- Explore across drug classes: GPS connects ideas across various therapeutic areas, allowing you to see how polymorph research is applied in multiple pharmaceutical contexts. You can also use GPS’s sort by relevance feature for filtering results based on what is more relevant to you.
The Global Patent Search helps R&D teams, formulation scientists, and patent professionals work more efficiently. It uncovers meaningful insights, clarifies the competitive landscape, and supports better decision-making during the development or defense of pharmaceutical innovations.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.