Every powerful medicine walks a fine line between helping and harming. That balance gets even harder when opioids are part of the story.
US12097187B2 is one of those rare patents that tries to walk that line right. It describes a drug designed to treat Irritable Bowel Syndrome with Diarrhea easing pain and bowel issues while keeping safety front and center.
Instead of relying on traditional opioids, it uses a dual-acting compound that soothes the gut without triggering the usual risks like constipation or misuse.
After a detailed patent prosecution, this patent is part of a litigation suit filed by Allergen, arguing that drugs of MSN Laboratories and Hetero USA infringe on the subject patent.In such disputes, opposing parties often challenge patent invalidity to prove that earlier work already disclosed similar claims.
But we are interested in the core technology part. So we used the Global Patent Search tool to see what came before, and what stands beside it.
What we found were five similar patents that shaped the same idea in different ways: how to make opioid-based treatments effective, safe, and hard to abuse.
Inside the Technology Behind US12097187B2
At the heart of this patent lies a smart idea: use the power of opioids while removing the danger that comes with them.
US12097187B2 describes a drug that targets two types of opioid receptors in the body. One helps control pain. The other, when blocked, keeps the gut moving normally. Together, they balance comfort and function, which is a rare combination in this category of drugs.
Here’s what makes the formulation stand out:
1. Balanced relief: It activates one receptor and blocks another, easing pain while avoiding constipation and other side effects.
2. Built for safety: The tablet cannot be crushed or altered easily. That small detail helps prevent misuse, a major concern for opioid-based medicines.
3. Designed for IBS-D: It focuses specifically on people with Irritable Bowel Syndrome with Diarrhea, improving both abdominal comfort and bowel control.
4. Tested and timed: The ideal dose is 75 mg, taken twice a day with food, based on clinical data showing clear symptom relief and safety.
In simple terms, this patent reimagines what an opioid-based therapy can be: strong enough to help, yet smart enough to protect.
Now that it is under legal scrutiny, understanding how other patents approached the same balance between power, safety, and control becomes even more important. That is where GPS reveals the full picture.
Recommended Read: Patent Audit of EP4344633B1: The design shift behind safer diabetes wearables
Exploring Similar Patents Through US12097187B2
Using the Global Patent Search tool, we identified five related patents that approach the same challenge from different angles.
Together, they reveal clear themes: smarter opioid use, safer formulations, targeted relief, and easier patient dosing.
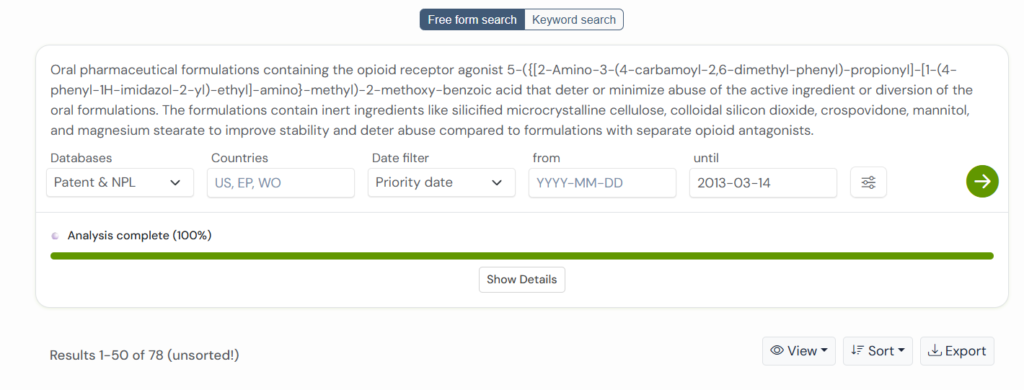
Each patent added something new, demonstrating non-obviousness through unique chemical or delivery improvements that shaped safer and more effective treatments.
Let’s look at each one to see how it connects to US12097187B2 and the larger story of controlled, patient-focused opioid therapy.
CA2359273A1 – Finding Balance Between Power and Safety
Filed in 2000, CA2359273A1 explores how to make opioid treatments safer without losing their effectiveness. It pairs an opioid with an α-agonist to balance strong pain relief with fewer side effects, using oral formulations that can release the drug either immediately or over time.
The connection with US12097187B2 lies in their shared philosophy: both patents aim to keep the therapeutic power of opioids while reducing misuse and discomfort.
CA2359273A1 focuses on chronic pain management, while US12097187B2 applies that same safety-first logic to treating IBS-D through dual receptor modulation. Together, they show how small shifts in formulation design can completely reshape patient safety outcomes.
The Broader Picture
CA2359273A1 set an important precedent for balancing efficacy with restraint. It marked a turning point in how researchers viewed opioid development. That is, not as a trade-off between relief and safety, but as an opportunity to achieve both.
That perspective paved the way for more advanced patents like US12097187B2, which transformed that balance into targeted gastrointestinal therapy.
ES2278647T3 – Making Opioid Treatment Easier on the Body
Filed in 2000 by Adolor Corp, ES2278647T3 tackles one of the biggest challenges in opioid therapy: the uncomfortable gastrointestinal side effects that come with it.
The invention combines opioids with peripherally selective antagonists to keep pain relief strong while easing symptoms like constipation, nausea, and slowed digestion.
The technical overlap with US12097187B2 lies in their shared effort to make opioid-based treatments more tolerable.
While ES2278647T3 focuses broadly on managing opioid-induced side effects, US12097187B2 takes that idea further, applying the same balance of efficacy and comfort to a specific condition: Irritable Bowel Syndrome with Diarrhea.
The Broader Picture
This patent represented a key step toward patient-centered opioid design. By separating therapeutic action from unwanted side effects, it demonstrated that comfort and control could coexist in a single formulation.
That thinking helped shape future drugs like US12097187B2, where targeted receptor control became the foundation for safer and smarter gastrointestinal therapies.
JP2003534380A – Building Flexibility Into Opioid Formulations
Also filed in 2001 by Grünenthal GmbH, JP2003534380A explores a combination of opioid compounds with secondary agents to create multi-target drug formulations.
The goal was simple but ambitious: make pain relief adaptable to different patient needs, delivery routes, and dosing patterns. The patent allowed for oral, intravenous, and subcutaneous administration, with doses ranging widely based on condition and response.
The technical overlap with US12097187B2 appears in their shared focus on oral delivery and twice-daily dosing.
Both aim to fine-tune how opioid-based drugs behave in the body, adjusting timing, strength, and receptor response to get consistent results without excess risk. While JP2003534380A stays broad in application, US12097187B2 narrows that flexibility into a targeted gastrointestinal therapy.
The Broader Picture
JP2003534380A reflects a key evolution in how drug developers began to see opioid treatment as a customizable framework. Its multi-route approach helped inspire future patents like US12097187B2, where precision dosing and controlled receptor targeting became central to designing safer, condition-specific medications.
Recommended Read: US8455531B2 and similar patents on improving amino acid absorption with nitrate blends
JPH1112166A – Refining Oral Opioid Delivery
JPH1112166A is another patent filed by the leader in pain management Grünenthal GmbH. The 1999 patent focuses on improving how opioid-based painkillers work when taken orally.
The patent introduced (+)-O-demethyltramadol as an active compound and explored ways to make it more effective for both acute and chronic pain management. By refining oral delivery and dosage control, it aimed to boost patient comfort without sacrificing potency.
The technical overlap with US12097187B2 lies in their shared focus on oral opioid formulations designed for consistent performance and patient compliance.
Both patents optimize how opioids are absorbed and maintained in the body, though US12097187B2 adds an extra layer of innovation, using receptor modulation to manage gastrointestinal function while reducing misuse potential.
The Broader Picture
JPH1112166A captures a moment when oral opioid delivery began evolving from simple pain control to precision-driven therapy.
Reducing preparation steps in clinical settings is a shared goal with US10869845B1, which removes dilution errors by offering ephedrine in a stable, ready-to-use injectable form.
TW476755B – When Opioid Science Began Looking Beyond Pain
This patent from Ortho-McNeil Pharmaceutical explored a family of compounds that could control delta-opioid receptors. The idea was to use these compounds to manage pain, diarrhea, and inflammation at once, proving that one receptor could influence both gut and neural functions.
The formulations were designed for oral use and tested across different therapeutic areas, from gastrointestinal disorders to central nervous system conditions. In essence, it took opioids out of their narrow pain-relief box and started viewing them as tools for broader regulation inside the body.
The overlap with US12097187B2 lies in that shift in thinking. TW476755B established the receptor groundwork, while US12097187B2 refined it into a focused therapy that delivers relief, protects against misuse, and keeps the gut functioning normally.
The Broader Picture
TW476755B represents the moment opioid science began expanding its purpose. It turned a pharmacological curiosity into a blueprint for safer, smarter treatments that understand how the gut and brain speak to each other.
US12097187B2 builds directly on that foundation, showing how far the science has come from those first exploratory steps.
Comparison Overview: How US12097187B2 Aligns with 5 Similar Patents
Each of these five patents solved a part of the same puzzle: how to make opioid-based treatments safer, smarter, and more reliable.
Some improved the chemistry. Others reimagined delivery or dosing.
When placed side by side with US12097187B2, they reveal how each step brought the field closer to what this patent achieves today: effective relief with built-in protection.
| Patent | Core Focus | Technical Overlap with US12097187B2 | The Broader Picture |
| CA2359273A1(Grünenthal GmbH, 2000) | Developed opioid formulations paired with α-agonists to maintain pain relief while reducing misuse and side effects. | Both aim to preserve opioid efficacy while reducing risks. CA2359273A1 focused on chronic pain, while US12097187B2 applied similar safety logic to IBS-D through dual receptor modulation. | Marked the start of seeing opioid design as a balance between power and protection — a mindset that US12097187B2 carried forward into gastrointestinal treatment. |
| ES2278647T3(Adolor Corp, 2000) | Combined opioids with selective antagonists to minimize GI side effects like constipation and nausea. | Both prioritize comfort alongside efficacy. ES2278647T3 eased opioid-induced side effects, while US12097187B2 targeted a specific GI disorder using the same balance of relief and tolerability. | Showed that patient-centric opioid therapy was possible — setting the stage for safer, symptom-specific drugs like US12097187B2. |
| JP2003534380A(Grünenthal GmbH, 2001) | Proposed flexible, multi-target opioid formulations adaptable across delivery routes and dosing schedules. | Shares focus on oral delivery and twice-daily dosing precision. JP2003534380A emphasized adaptability; US12097187B2 transformed that flexibility into a controlled, condition-specific treatment. | Reflected a move toward personalized opioid therapies, inspiring later innovations in dosage control and receptor targeting. |
| JPH1112166A(Grünenthal GmbH, 1999) | Improved oral administration of (+)-O-demethyltramadol for acute and chronic pain management. | Both refined oral opioid formulations for better absorption and consistency. US12097187B2 extended that refinement to gut-focused relief and built-in abuse resistance. | Captured a turning point when oral delivery became about precision and predictability, paving the way for advanced receptor-based GI therapies. |
| TW476755B(Ortho-McNeil Pharmaceutical, 1999) | Explored delta-opioid receptor compounds for pain, inflammation, and diarrhea management. | Both target δ-opioid receptors for gut regulation. TW476755B laid the biochemical foundation; US12097187B2 expanded it into a safer, dual-acting treatment. | Signaled when opioid research began linking gut and brain responses — a connection that US12097187B2 later refined into modern, misuse-resistant IBS-D therapy. |
Seen together, these patents form a timeline of steady progress: from early ideas about receptor balance to modern, patient-focused opioid formulations.
They don’t compete with US12097187B2 as much as they complete its story. Each one adds a building block that helps explain how the science of safety evolved.
Related Read: If you want to explore how drug formulations like this are discovered today, our article on AI-driven drug discovery highlights where pharmaceutical innovation is heading.
But connecting these dots isn’t easy. That’s where tools like Global Patent Search comes in.
Let’s explore how to use GPS to surface these insights and make sense of complex patent landscapes like this one.
Finding the Hidden Links with Global Patent Search Tool
When a pharmaceutical patent like US12097187B2 becomes the center of litigation, knowing what surrounds it can be just as important as knowing what it claims.
GPS helps uncover the full ecosystem around a patent: the similar formulations, dosing methods, and chemical strategies that define its technical neighborhood.
It’s designed to help you see patterns across filings that might otherwise take weeks of manual digging to uncover.
Here’s how GPS helps you do that:
Start with the patent or idea: Enter a patent number like US12097187B2 or a concept such as “dual-acting opioid therapy” or “oral IBS-D formulation.” GPS understands both keyword based and natural-language inputs, surfacing patents that share core scientific intent.
Get excerpts, not heavy text: GPS now shows exact snippets from similar patents, such as where the claim or description actually overlap, so you can skip pages of legal text and focus only on the parts that matter.
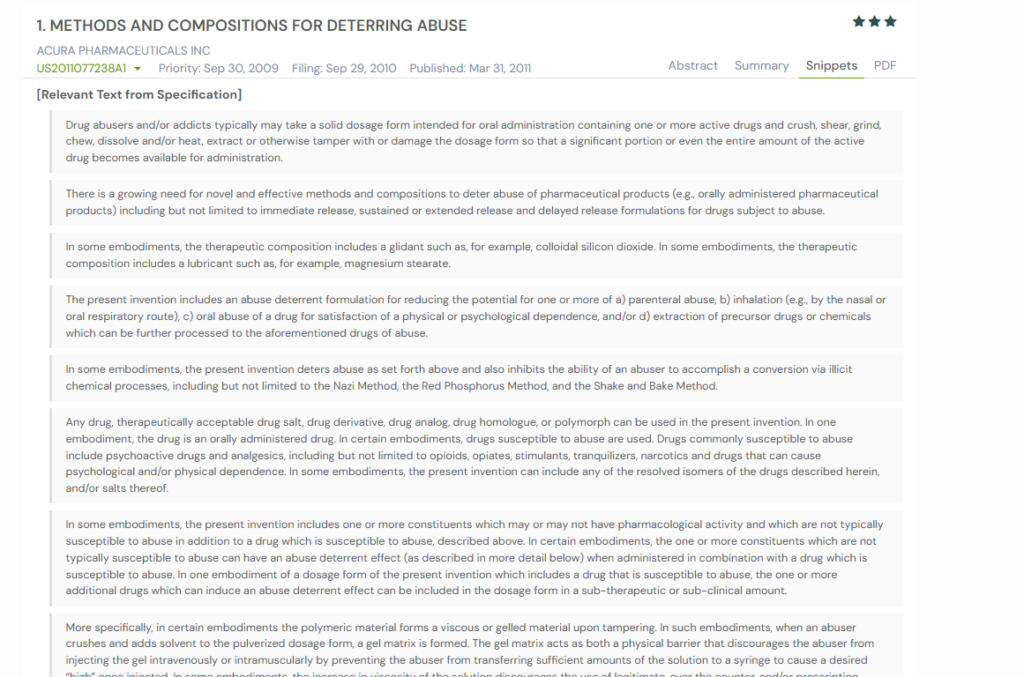
Once you think a reference matches you need, you can open the full document and analyze further.
Turn insight into strategy: Whether you’re defending exclusivity, ensuring freedom to operate, or identifying R&D directions, GPS gives you the technical and contextual clarity you need to act with confidence.
By linking patents that speak the same scientific language, GPS helps you understand not just what was invented, but how the field itself evolved.
In complex domains like opioid modulation or gastrointestinal therapy, that broader view can make all the difference. Try the tool today to find out how the tool works!
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.