Diluting ephedrine looks easy in theory, but in real hospital settings, it often becomes the slowest step in the entire workflow. A concentrated vial needs to be mixed, the pH needs to stay within range, and the final solution has to be prepared quickly without mistakes. This routine step creates delays at moments where time should be spent on the patient, not on the preparation.
US10869845B1 was created to solve this long-standing issue. Instead of sending clinicians a strong solution that must be diluted, it shows how ephedrine can be made in a ready-to-use form that stays stable for months.
The formulation holds its pH, avoids drifting outside acceptable limits, and removes unnecessary steps from the treatment process. Its importance is clear from the amount of attention it has drawn, including a dispute between PH Health Limited and Fresenius Kabi USA.
With the Global Patent Search(GPS) tool, we wanted to trace how this idea evolved, explore similar inventions, and understand why this approach finally makes ephedrine easier to use.
What US10869845B1 Is Actually Solving
To understand this patent, it helps to look closely at what really goes wrong when ephedrine is diluted for clinical use.
The drug itself is effective, but once diluted to a usable strength, its pH becomes unstable. Even small shifts can push it outside the FDA-recommended range, which means the solution has to be checked, adjusted, or used immediately. This creates a product that works on paper but struggles to stay reliable in real conditions.
US10869845B1, filed on January 2020, focuses on fixing that gap. It explains how ephedrine can be formulated at about 4 to 6 mg per milliliter with a controlled pH between 4.5 and 5.0. The inventors discovered that when acetic acid is used as the pH adjuster, and the formulation is balanced correctly, the solution stays stable for months without drifting.
The result is an Ephedrine solution that can be stored, handled, and used without extra preparation steps or constant pH adjustments.
Key Features of US10869845B1
The below features explain how this formulation makes ephedrine stable and ready to use without extra preparation.
- The formulation holds ephedrine at a practical 4 to 6 mg per milliliter concentration that clinicians can use directly.
- A carefully maintained pH window between 4.5 and 5.0 keeps the solution within the ideal stability range.
- Acetic acid is used to adjust the pH, creating a gentler environment that prevents sudden shifts.
- A true single-phase system ensures the entire mixture remains uniform without forming separate layers.
- Optional components like sodium chloride or mild buffers can be added without disrupting long-term stability.
All of this adds up to a formulation that removes unnecessary steps and gives hospitals a version of ephedrine they can trust.
If you’re interested in how process chemistry shapes modern drug manufacturing, our patent audit of US12128039B2 shows how improved synthesis and crystallization methods help create cleaner, more stable quinoline compounds used in kinase-targeted cancer therapies.
How Earlier Patents Shaped the Path to US10869845B1
To see why US10869845B1 matters, it helps to look at the inventions that came before it. Different teams tried different approaches to keep diluted ephedrine stable, and each idea moved the field forward in its own way.
When you review these earlier filings through the Global Patent Search tool, you can see how the thinking around pH control, storage conditions, and formulation design gradually evolved. Together, they create the backdrop that makes the stability achieved in this patent stand out.
Let’s explore some of them.
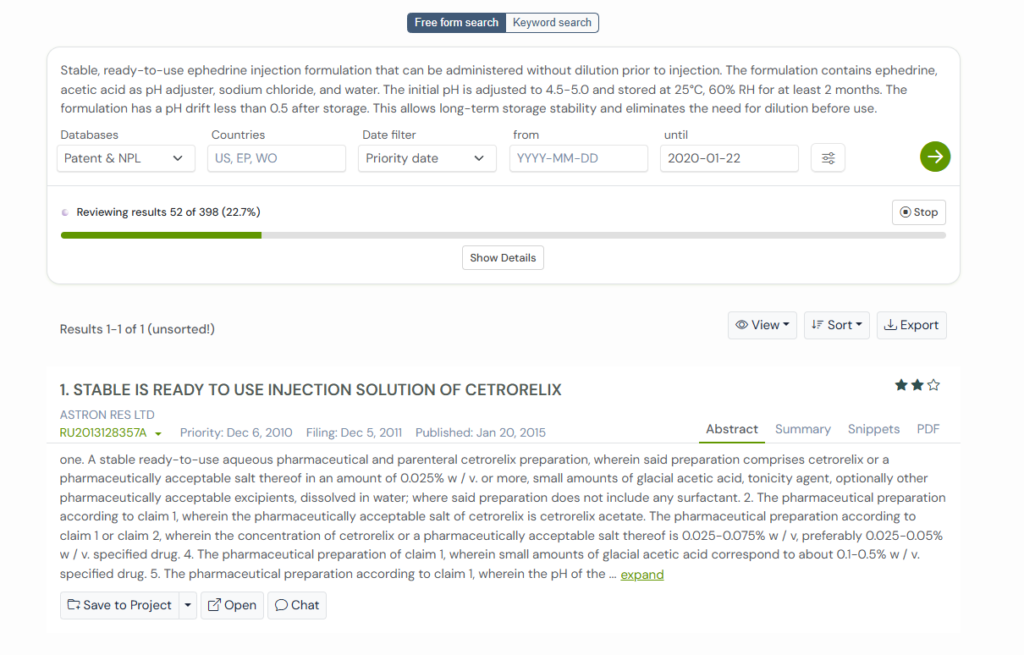
1. EP3305294A1
EP3305294A1 was filed by Kyorin Pharmaceutical with a focus on a challenge that quietly affects many injectable drugs. When two liquid components are mixed, the overall stability depends heavily on keeping the pH within a tight range.
Most formulations drift because their acids, bases, and buffers do not hold steady once combined with salts, sugars, or carriers. This patent addresses that issue by mapping out a wide set of pH adjusters and explaining how each one helps maintain balance in different solution environments.
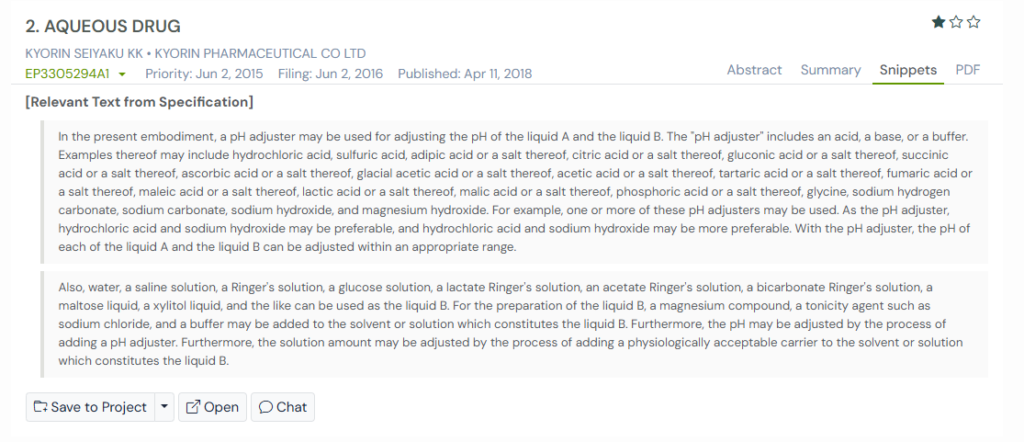
The inventors approached the problem by giving formulators flexibility. They showed that acids like acetic, citric, or hydrochloric acid, and bases like sodium hydroxide, can be selected depending on how the final mixture behaves. By controlling pH in both liquid A and liquid B, they created a foundation for more predictable aqueous drug compositions.
This matters for US10869845B1 because both inventions treat pH control as the key to stability. EP3305294A1 focuses on identifying the right adjusters, while US10869845B1 uses a very specific combination and concentration to keep ephedrine stable over long storage periods.
The Bigger Picture
The patent reflects a shift toward fine tuning pH rather than relying on heavy buffers or complex additives. It shows how simple acid-base choices can influence long term behavior. Through references surfaced by GPS, you can see how this earlier work laid useful groundwork for ready to use ephedrine formulations that depend on precise pH control.
For a look at how purification challenges shape injectable-grade chemicals, our analysis of US12304813B2 explains how sodium thiosulfate is cleaned, stabilized, and tested without breaking down during analysis. It shows how industrial ingredients are transformed into pharmaceutical-grade materials suitable for modern medical use.
2. US2014005219A1
US2014005219A1 from Mallinckrodt focuses on improving the long-term pH stability of intrathecal hydromorphone solutions. Hydromorphone tends to drift in pH during storage, but the inventors discovered that using minimal additives and selecting gentle acids helps the solution stay within a tight pH range for months or even years.
Another important insight from this patent is that a cleaner formulation often performs better over time. The inventors proved that by removing certain buffers, the pH stayed closer to physiological levels and impurities remained low. Their stability data across different temperatures reinforces how powerful small formulation choices can be.
This work directly connects to US10869845B1 because both patents show that stability comes from controlling pH with simple components rather than relying on heavy buffers or complex excipients.
The Bigger Picture
This patent highlights a broader trend in injectable drug design where stability is achieved through precise pH control and minimal formulation complexity. It sets a strong foundation for ready-to-use solutions like the one described in US10869845B1, which applies the same principle to ephedrine.
Our patent audit of EP3175863B1 shows how researchers extend the half-life of therapeutic peptides using different approaches, allowing the medicines to remain active for longer in the bloodstream for certain treatments.
3. KR20020048400A
KR20020048400A, filed in 2002 by Orion Corporation, focuses on improving the stability of levosimendan in aqueous injectable formulations. The patent aims to create a formulation that remains reliable during storage while being suitable for direct intravenous use.

The patent centers on careful pH control. By using selected organic acids or buffer systems and keeping the pH at 5 or below, the formulation avoids common degradation pathways. Acids like citric, tartaric, and lactic acid are highlighted for their ability to support long-term chemical stability.
This connects to US10869845B1 because both patents show that dependable, ready-to-use injectables are built on precise pH management. While KR20020048400A focuses on levosimendan, the same principle is applied to ephedrine to prevent pH drift and maintain strength over time.
The Bigger Picture
This patent reflects a broader shift in formulation science where stability is achieved through minimal, well-chosen components rather than complex additives. It helped establish the idea that the right pH environment can protect a drug more effectively than heavy buffering systems.
4. CN103417475A
CN103417475A, filed in 2013 by Shenzhen Hybio Pharmaceutical, explored why some barusiban injections lost purity during storage. Older formulations used simple acetate systems, but these mixtures broke down quickly. After a few months, the drug lost strength, impurities increased, and the solution no longer met quality standards.
To fix this, the inventors tested different pH regulators. They tried phosphoric acid, citric acid, hydrochloric acid, and balanced acid–base pairs like phosphoric acid with disodium hydrogen phosphate or acetic acid with sodium acetate. These combinations created a more stable environment where the pH stayed steady even during long term storage.
This connects directly to US10869845B1 because both patents show the same principle in very simple terms. If the pH stays stable, the drug stays stable. CN103417475A proves this with barusiban, and US10869845B1 applies the same idea to ephedrine.
The Bigger Picture
This patent shows that stability often depends on choosing the right pH system rather than adding more ingredients. When the environment is controlled, even sensitive injections can remain usable for long periods.
While applied in a different industry, US11105426B2 follows a similar design philosophy of eliminating internal failure points that accelerate wear under harsh conditions.
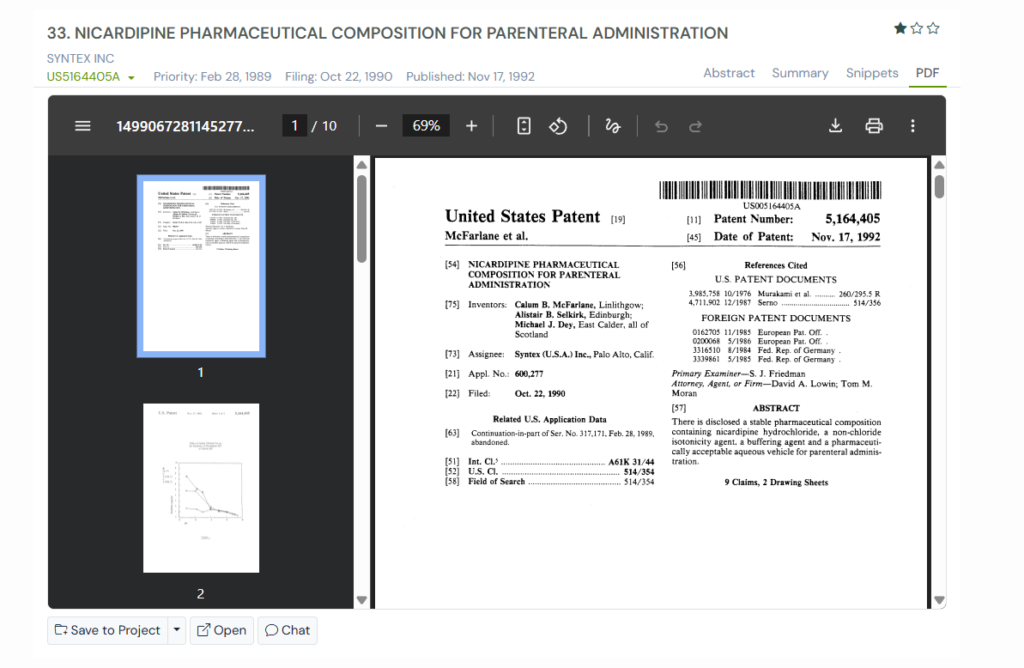
5. US5164405A
US5164405A, filed in 1990, dealt with a long-standing problem in making injectable nicardipine. When nicardipine hydrochloride is mixed in water, part of it converts into its free base, which is poorly soluble and forms a sticky yellow precipitate.
This makes the solution unstable, difficult to prepare, and unsuitable for direct clinical use. The patent works on creating a stable, clear formulation that stays intact during storage and dilution.
The inventors discovered that nicardipine becomes far more stable when the formulation uses non-chloride isotonicity agents and a carefully chosen buffer system inside a fully aqueous vehicle. The right balance of pH, ionic strength, and solvent environment prevents the formation of the free base and stops precipitation. As a result, the drug dissolves properly and remains clear even when diluted further for infusion.
This connects to US10869845B1 in a very simple way: both patents show that stability problems in injectables usually come from pH drift and unwanted chemical changes in water.
The Bigger Picture
This patent reinforces a core idea across injectable formulations: if the chemical environment is controlled correctly, even sensitive or poorly soluble drugs can become reliable, stable, and safe for direct administration.
How These Patents Line Up Side by Side
A quick comparison makes it easier to see how each invention approaches stability in injectable formulations. Every patent uses its own mix of pH control, excipients, and design choices, and placing them together highlights what makes US10869845B1 unique.
| Patent Number | Filed In (Year) | Focus of the Invention | Key Stability Strategy | Why It Matters in Understanding Stability |
| EP3305294A1 | 2018 | Stabilizing multi-component aqueous drug solutions | Flexible acid–base adjusters to maintain a stable pH in mixed liquids | Shows how choosing the right pH adjusters (rather than heavy buffers) creates predictable long-term stability |
| US2014005219A1 | 2014 | Long-term stability of intrathecal hydromorphone | Minimal excipients + gentle acids to reduce pH drift and impurities | Demonstrates that simpler, low-additive formulations often stay stable longer than heavily buffered ones |
| KR20020048400A | 2002 | Stable levosimendan injectable formulation | Organic acids and low-pH buffer systems to limit degradation | Establishes that sensitive injectables perform better when kept in a controlled acidic environment |
| CN103417475A | 2013 | Preventing barusiban injection degradation during storage | Paired pH regulators (acid + conjugate base) for long-term pH consistency | Shows how impurity growth can be reduced by choosing more resilient pH systems rather than adding extra excipients |
| US5164405A | 1990 | Stable nicardipine injectable without precipitation | Non-chloride isotonicity agents + balanced buffers to prevent free-base formation | Proves that instability often comes from the vehicle environment, and tuning ionic balance prevents precipitation |
Why GPS Matters When You Study Patents Like This
When you look at stability patents one by one, each feels like a standalone solution. One focuses on pH drift, another removes buffers, another varies excipients, and yet another adjusts concentration to achieve longer shelf life.
But once you place them next to each other, a much bigger story appears. You start to see how different teams solved similar formulation problems using very different choices.
This is exactly where the Global Patent Search tool becomes useful. Instead of digging through long PDFs on traditional patent search tools, GPS pulls the right references together so you can understand how an idea evolved across companies, molecules, and formulation strategies.
Searching with GPS is easy. All you have to do is:
- Enter a patent number and the AI-powered search engine instantly surfaces related work in the same technical space.
- Scan short, clear snippets that show what each patent or NPL is trying to fix.
- Jump into the full text whenever you want to compare claims or formulation logic.
- Notice patterns like recurring pH windows, common excipients, or stability conditions. You can use the sort by relevance feature to surface more patents related to an area you are focusing on.
- Find how newer inventions innovate further on what earlier patents attempted.
GPS turns scattered pharmaceutical innovations into a single, connected storyline. You get context, clarity, and a faster way to understand how a solution like US10869845B1 fits into the larger landscape of injectable-drug stability.
Whenever you want to explore more patents with meaningful connections, GPS is where that journey becomes easier.
Frequently Asked Questions
1. Why do diluted injectable drugs often become unstable?
Many diluted formulations lose stability because lowering the concentration exposes the drug to faster degradation. Factors like pH shifts, dissolved oxygen, light exposure, and reactive excipients can accelerate the process.
2. What makes pH control so important in injectable solutions?
Even a minor pH change can affect how quickly a drug degrades, forms impurities, or loses potency. A tightly controlled pH range helps maintain chemical stability throughout storage and transport.
3. What is a ready-to-use injectable formulation?
It is a pre-diluted, sterilized solution that can be administered directly without mixing at the hospital. This avoids preparation errors and reduces contamination risk.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.