People with clogged arteries in the heart or legs live with a constant risk. Even on standard care, a sudden heart attack, stroke, or limb emergency can still happen. The challenge is cutting that risk without causing unsafe bleeding. A close pharmaceutical parallel is US12245996B2, which demonstrates how careful excipient and pH choices keep critical injectables stable and ready for emergency use.
US10828310B2 proposes a clear, practical answer: combine two small daily doses comprising rivaroxaban (a targeted blood thinner) twice a day and aspirin once a day. This lowers major cardiovascular events in people with coronary artery disease or peripheral artery disease.
The idea is simple: use two medicines that prevent clots in different ways, at carefully chosen low doses, to do more together than aspirin alone.
The patent is currently cited in cases brought by Bayer Pharma against Lupin Ltd., Aurobindo Pharma, and Apotex Inc., but our focus here is the science. We wanted to see how this invention fits into the wider search for safer cardiovascular therapies.
Using the Global Patent Search (GPS) tool, we examined similar patents to see how other researchers are approaching the same life-threatening problem.
Understanding Patent US10828310B2
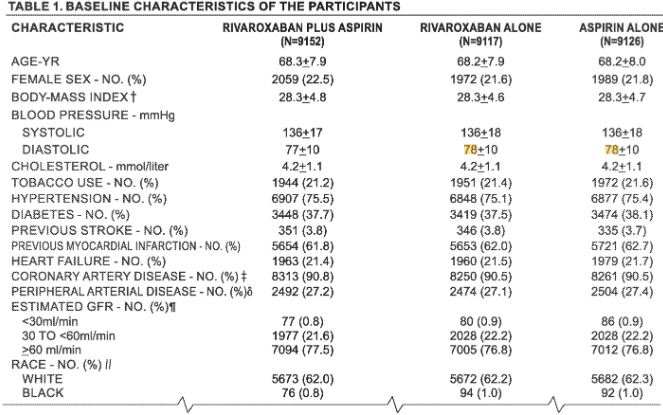
US10828310B2 describes a treatment that helps prevent heart attacks, strokes, and related events. It focuses on patients with coronary artery disease (CAD) or peripheral artery disease (PAD), conditions that affect blood flow.
The invention combines two well-known drugs: rivaroxaban, a blood thinner, and aspirin, an antiplatelet, to reduce health risks. This combination is designed for long-term use and aims to provide enhanced protection compared to aspirin alone.
Source Google Patents
The Key Features Of This Patent Are
1. 2.5 mg rivaroxaban is given twice a day.
2. 75–100 mg aspirin is given once daily.
3. Used for treating patients with CAD and/or PAD.
4. Reduces major cardiovascular events like stroke, heart attack, or cardiovascular death.
The patent also covers methods to reduce major limb events, such as acute limb ischemia. It defines specific dose ranges and hazard ratios proven in large-scale clinical trials. The treatment can be delivered as a single product or as separate pills. It also includes the idea of combining this therapy with other standard heart medications.
This invention builds on existing therapies but shows improved outcomes in real-world clinical studies. By combining two low-dose drugs, the method balances effectiveness with safety, something many older treatments failed to achieve.
Related Read: See how US12128039B2 enhances cancer therapies by ensuring drug purity and stability.
Similar Patents As US10828310B2
To explore how others have approached the same medical challenge, we used the Global Patent Search platform to uncover similar patents. These references reveal early attempts at combining anticoagulants and antiplatelet drugs to prevent major cardiovascular events.
While US10828310B2 focuses on a specific fixed-dose combination of rivaroxaban and aspirin, the following patents highlight broader or earlier strategies that align with this goal.
1. WO9856365A1
This international patent, WO9856365A1, published in 1998, describes a treatment for arterial clots using a Factor Xa inhibitor, either alone or with a platelet aggregation inhibitor. It outlines how blocking Factor Xa helps stop clot formation, especially in high-risk arteries.
The invention proposes combinations that may include various anticoagulants and antiplatelet drugs delivered through oral or injectable routes. The patent also gives flexible dosage guidance, highlighting the potential benefits of dual-action therapy.
Below, we have added snapshots from the GPS tool displaying the overlap from the specification for similar patents.
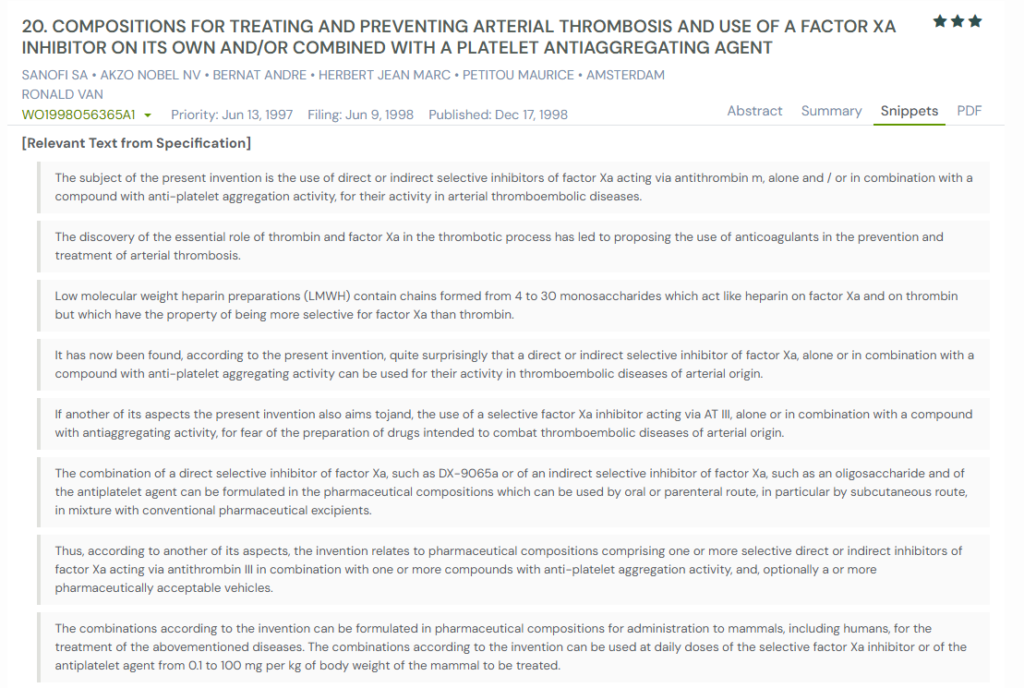
What This Patent Introduces To The Landscape
- Use of direct or indirect Factor Xa inhibitors for arterial thrombosis
- Combination with antiplatelet agents such as aspirin
- Focus on arterial thromboembolic diseases like heart attack or stroke
- Dosage range from 0.1 to 100 mg/kg body weight per day
- Flexible delivery methods: oral, subcutaneous, or intravenous
- Preferred combinations: 15–25 mg Factor Xa inhibitor + 10–30 mg antiplatelet agent
- Pharmaceutical compositions include excipients for stability and delivery
- Early proposal of dual-action therapy targeting both clotting and platelet activity
How It Connects To US10828310B2
- Both target arterial disease prevention using dual-drug combinations.
- US10828310B2 uses rivaroxaban (Factor Xa inhibitor) + aspirin (antiplatelet)
- WO9856365A1 proposes a similar dual-pathway inhibition strategy
- Each covers pharmaceutical compositions combining both drug classes
- Focus on preventing events like stroke, heart attack, and clot-related complications
- Both include clinical dose guidance and delivery route flexibility.
Why This Matters
This early reference introduces the idea of combining Factor Xa inhibitors with antiplatelet agents. While less specific in terms of drugs and dosages, it laid the groundwork for future therapies, such as US10828310B2.
The concepts of dual therapy, arterial clot prevention, and dosage flexibility remain highly relevant. WO9856365A1 supports the long-standing interest in combination strategies to improve cardiovascular outcomes.
Recommended Read: Explore how US9326945B2 and other patents in the Factor Xa inhibitor space tackle similar challenges in dosing, absorption, and therapeutic targeting for cardiovascular care.
2. EP1528061A1
This European patent, EP1528061A1, published in 2005, presents a new class of compounds that block Factor Xa. Factor Xa is a key protein in the blood-clotting chain. By stopping its activity, these compounds help prevent dangerous blood clots.
The invention describes how these inhibitors can treat or prevent heart attacks, strokes, and other clot-related issues. It also suggests combining them with drugs like aspirin to improve effectiveness in high-risk patients.
What This Patent Introduces To The Landscape
- Strong Factor Xa inhibition to prevent clot formation
- Use in treating or preventing coronary and cerebrovascular conditions
- Application for both arterial and venous thrombosis
- Effectiveness in surgery-related clot prevention (e.g., angioplasty, heart valve procedures)
- Potential use in preventing clotting in stored blood samples
- Combination with antiplatelet agents like aspirin for enhanced protection
- Applicability across various cardiovascular emergencies, including stroke and pulmonary embolism
- Antithrombotic and anticoagulant action demonstrated in animal models
How It Connects To US10828310B2
- Both focus on Factor Xa inhibition to prevent life-threatening cardiovascular events
- US10828310B2 uses rivaroxaban, while this patent covers a broader set of compounds
- Each considers dual-drug strategies involving antiplatelet agents like aspirin
- The target diseases are nearly identical: heart attacks, strokes, and blood clots
- Both patents support combining anticoagulant therapy with other heart-protective treatments
Why This Matters
This patent expands the toolkit for blocking Factor Xa, a proven strategy in clot prevention. Like US10828310B2, it aims to reduce risks for patients with serious vascular conditions. Its inclusion of combination therapy and broad medical use cases strengthens the case for dual-pathway treatment approaches.
While treating different conditions, US12029779B2 and similar patents share a common focus with this patent on combination therapies. Both blend multiple active agents to enhance long-term treatment effectiveness.
3. WO2006108709A1
This international patent, WO2006108709A1, published in 2006, introduces a class of chemical compounds that work by blocking Factor Xa. These inhibitors are meant to treat or prevent serious vascular conditions linked to blood clots.
The compounds are designed for use in heart disease, stroke, deep vein thrombosis, and related conditions. The invention also outlines the potential for combining these Factor Xa inhibitors with other therapies, such as aspirin, anti-inflammatories, or antihypertensive drugs, for better results.
What This Patent Introduces To The Landscape
- Novel Factor Xa inhibitors based on sulfonylamino-pyrrolidine-2-one structure
- Treatment of acute coronary syndromes and unstable angina
- Application in preventing stroke, pulmonary embolism, and deep vein thrombosis
- Use in both primary and secondary prevention of myocardial infarction
- Protection against restenosis and vessel narrowing after angioplasty
- Utility in managing thromboembolism linked to atrial fibrillation
- Combinations with aspirin and other anticoagulant or antihypertensive agents
- Potential use in blood storage, dialysis, and prosthetic device coatings
- Broader application across inflammatory, neurodegenerative, and atherosclerotic conditions
- Dose adjustments when combined with other therapeutic agents
How It Connects To US10828310B2
- Both use Factor Xa inhibition to prevent clot-related cardiovascular events
- Each patent discusses combination therapy using antiplatelet agents like aspirin
- US10828310B2 focuses on fixed-dose rivaroxaban + aspirin, while this patent introduces new molecules
- Both aim to improve outcomes in patients at risk of heart attack or stroke
- Each covers the use of the compounds in various delivery forms and combinations
Why This Matters
This patent expands the range of chemical tools for targeting Factor Xa. It strengthens the case for dual-drug strategies and supports broader medical uses. Like US10828310B2, it reflects a growing trend in personalized anticoagulant therapy that balances safety and efficacy.
Related Read: See how US12122789B2 tackles stability in rare-disease treatments, much like cardiovascular drug patents that improve reliability through formulation breakthroughs.
4. WO2007008143A1
This international patent, WO2007008143A1, published in 2007, introduces a group of heterocyclic compounds designed to block Factor Xa. These compounds act as anticoagulants and are intended to prevent or treat blood clot-related problems.
The invention highlights that these inhibitors are selective for Factor Xa, reducing side effects compared to broader anticoagulants. The patent also supports using these compounds alone or combined with other drugs like aspirin, heparin, or clot-busting agents.

What This Patent Introduces To The Landscape
- Selective Factor Xa inhibitors based on heterocyclic sulfonamide structures
- Targeted treatment of coronary and cerebrovascular conditions
- Prevention of myocardial infarction, stroke, and clot-related complications
- Effective against arterial and venous thrombosis
- Use in restenosis prevention post-angioplasty or bypass surgery
- Application in surgeries involving artificial valves or blood circulation support
- Use in blood storage or dialysis settings to prevent coagulation
- Compatibility with a wide range of antithrombotic agents
- Combinations with aspirin, heparin, or thrombin inhibitors for added benefit
- Design flexibility to fit various therapeutic settings and patient needs
How It Connects To US10828310B2
- Both target Factor Xa to stop clot formation and reduce cardiovascular risks
- US10828310B2 uses rivaroxaban, while this patent introduces a new class of molecules
- Each includes the option to combine with aspirin for better outcomes
- Both cover multiple delivery settings, from standalone therapy to combination use
- The patents emphasize the precision treatment of patients prone to clotting disorders
Why This Matters
This patent builds on the Factor Xa strategy by introducing selective compounds with broad utility. Like US10828310B2, it supports safer and more targeted blood-thinning approaches, especially for high-risk cardiovascular patients.
5. US2004029874A1
This US patent application, US2004029874A1, published in 2004, describes a class of aromatic amides that act as potent and selective Factor Xa inhibitors. These compounds are designed to deliver effective anticoagulation in mammals without interfering significantly with the natural clot-lysis process.
The invention proposes oral formulations with high bioavailability, intended for the treatment and prevention of thromboembolic disorders. This includes deep vein thrombosis, pulmonary embolism, myocardial infarction, and ischemic stroke. The compounds may also be used in conjunction with aspirin or thrombolytics for enhanced therapeutic outcomes.
What This Patent Introduces To The Landscape
- Aromatic amides as selective Factor Xa inhibitors
- High oral bioavailability, enabling convenient administration
- Treatment and prevention of thromboembolic disorders, including stroke and myocardial infarction
- Minimal interference with fibrinolysis mechanisms
- Use in hypercoagulable states post-surgery (e.g., angioplasty, bypass)
- Compatibility with aspirin and thrombolytic agents
- Suitable for both therapeutic and prophylactic applications
- Effective in both in vivo (human/animal) and in vitro settings
- Offers adjunctive use with clot-lysing therapies
- Includes pharmaceutical compositions with acceptable carriers and excipients
How It Connects To US10828310B2
- Both inventions focus on targeting Factor Xa as the key anticoagulation strategy
- US10828310B2 uses rivaroxaban, while this patent introduces aromatic amides as alternative inhibitors
- Each allows for combination therapy, including with aspirin or thrombolytics
- Emphasis on selectivity and minimizing bleeding risks appears in both disclosures
- Shared application in cardiovascular and thrombotic diseases
Why This Matters
This patent reinforces the value of Factor Xa as a druggable target and presents aromatic amides as a promising alternative to existing anticoagulants. It aligns closely with US10828310B2 in its therapeutic goals and introduces options for oral delivery and combinatory use with clot-dissolving agents.
Related Read: See how US12245996B2 stabilizes emergency norepinephrine injections for critical care, complementing the way US10828310B2 refines cardiovascular therapies with dual-drug precision.
How To Find Similar Patents Using Global Patent Search
If you are reviewing a patent like US10828310B2, it is helpful to see how it fits within the surrounding field. The Global Patent Search tool is built to locate similar patents, not ideas or innovations, but legally defined filings that share technical claims or applications.
1. Start with the patent number or keywords: Enter US10828310B2 into the search bar. You can also use related terms like “Factor Xa inhibitor,” “combination therapy,” or “anticoagulant formulations.”
2. Use snippets to get a quick sense of relevance: GPS shows short excerpts that highlight what each related patent is about, whether it is drug combinations, mechanisms of action, or clinical applications.
3. Look for claim overlaps and distinctions: Some filings might use similar compounds but target different conditions or dosing strategies.
4. Accelerate cross-domain insights: Whether you are working on antithrombotic therapies or exploring fixed-dose drug combinations, GPS connects you with similar pharmaceutical patents, even when they target different diseases or mechanisms.
Using Global Patent Search helps you stay informed and make smarter decisions, whether you are researching for development, legal review, or competitive analysis. It is a practical way to stay a step ahead in any patent-heavy industry. Try the tool today!
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.