EP4241767B1 isn’t just another entry in the biotech patent landscape. This European patent addresses one of the most pressing frontiers: the efficient delivery of therapeutic nucleic acids. Assigned to Arbutus Biopharma, the patent describes lipid particles that do more than transport. They increase fluidity, improve uptake, and reduce toxicity in gene-based treatments.
This patent has recently drawn attention from Moderna and several of its European affiliates. While the legal developments are ongoing, our focus stays technical. What matters here is how this patent fits into a broader research ecosystem of delivery systems and lipid-based carriers.
Using the Global Patent Search (GPS) tool, we explore structurally and functionally similar disclosures. No legal arguments, just data-backed feature analysis. If you’re in IP strategy, drug development, or bio-nanotech research, this is a detailed look you’ll want to examine.
Understanding Patent EP4241767B1
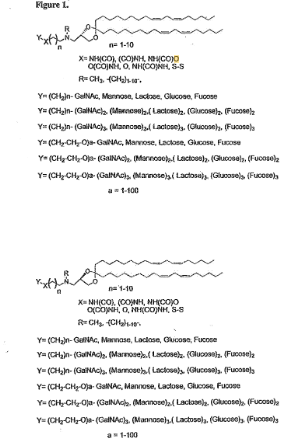
EP4241767B1 discloses novel lipid particles formulated for the delivery of therapeutic nucleic acids. These particles contain specific cationic lipids optimized for in vivo gene‑based therapies.
The invention aims to boost delivery efficiency, enhance membrane fluidity, increase nucleic acid uptake, and reduce toxicity. These formulation principles align closely with trends seen across today’s synthetic biology companies working on RNA and gene-based delivery systems.
Source: Google Patents
Its Four Key Features Are
#1. Cationic lipid with optimized unsaturated acyl chains – These lipids are designed to improve membrane fluidity and facilitate better cellular uptake.
#2. Encapsulation of therapeutic nucleic acids – The lipid particles are capable of carrying siRNA, mRNA, plasmids, or immunostimulatory oligonucleotides within their structure.
#3. Inclusion of neutral lipids and aggregation-reducing lipids – Components such as PEG-lipids are added to increase particle stability and extend circulation time in the bloodstream.
#4. Formulations designed to increase therapeutic index – The compositions aim to deliver enhanced therapeutic activity while minimizing systemic toxicity.
This patent sets a benchmark for lipid‑mediated delivery systems in gene therapy and RNAi platforms. It offers structured design principles for delivery carriers that strike a balance between efficacy and tolerability.
Fun Fact: Lipid structures evolve across therapies. EP2279254B1 Patent Audit details balanced lipid ratios for RNA delivery, complementing gene therapy patents that also rely on lipid carriers for safe intracellular transport.
Similar Patents As EP4241767B1
To explore the innovation landscape surrounding EP4241767B1, we ran the patent through the Global Patent Search tool. Below is a quick glimpse of the GPS tool in action:
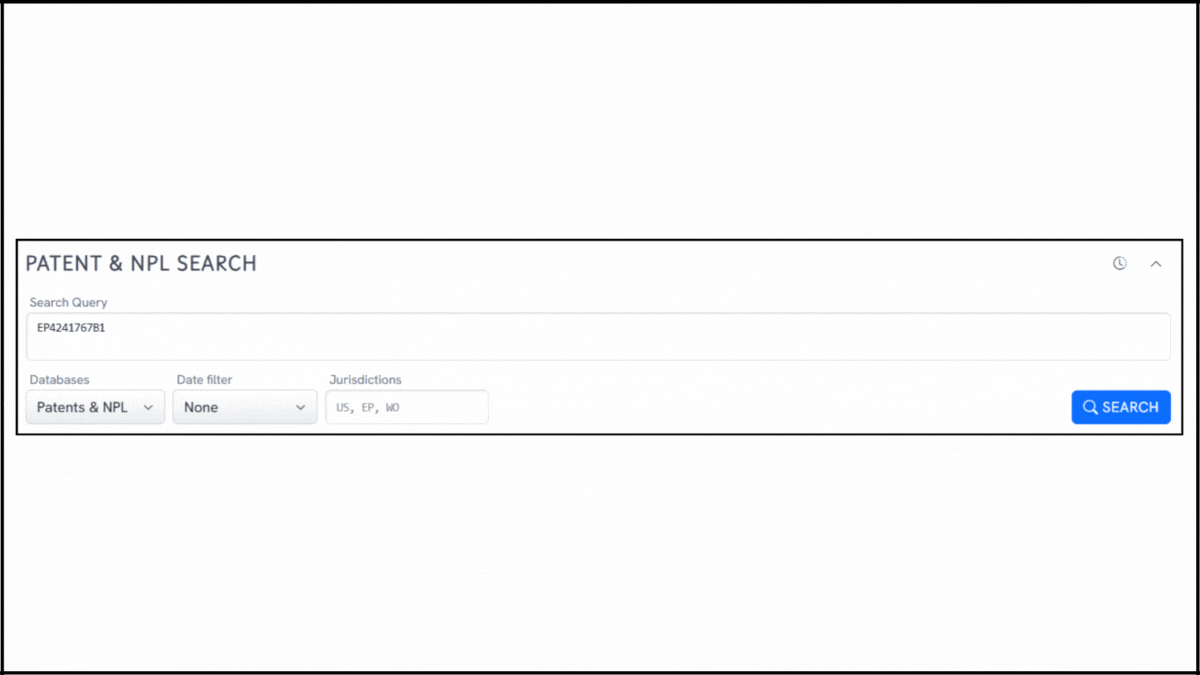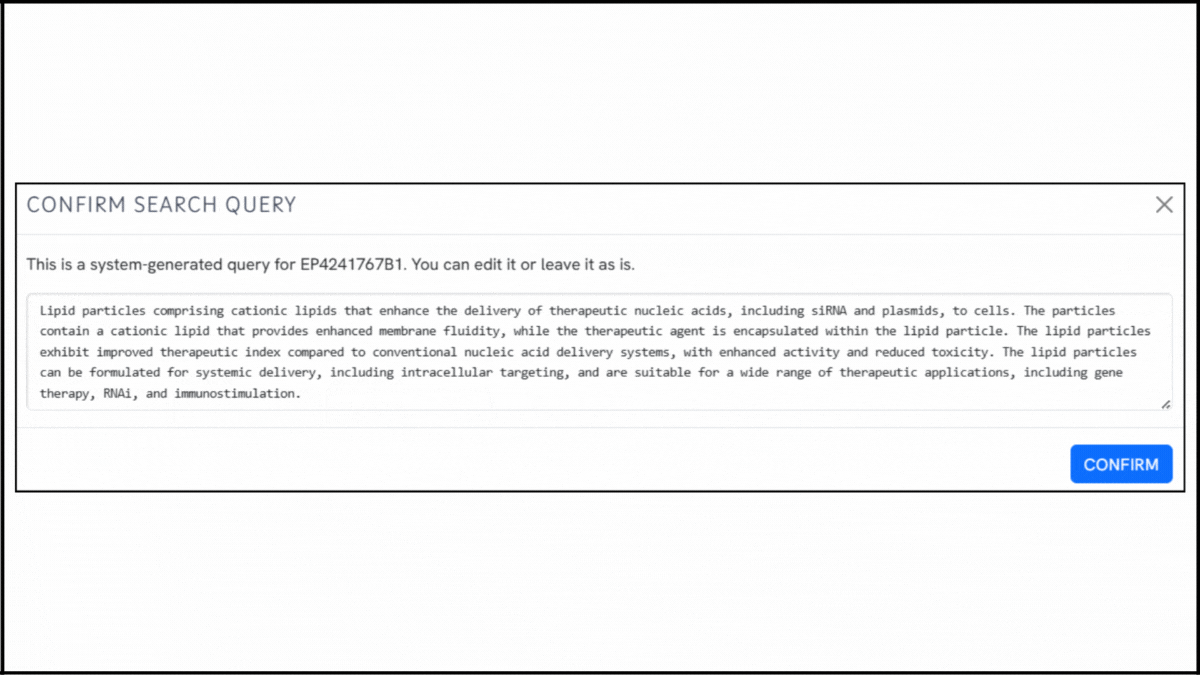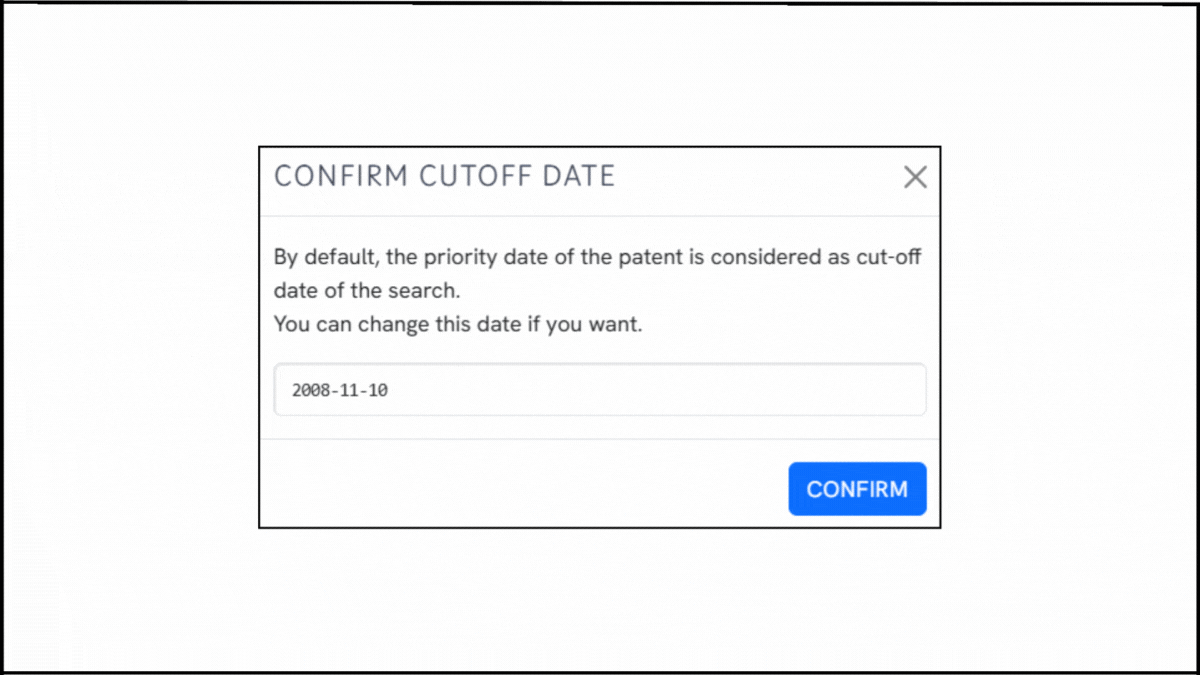
Source: Global Patent Search
This analysis surfaced a list of related patents that share technical similarities in lipid structure, nucleic acid encapsulation, and systemic delivery design. Below, we highlight five of these references that reflect comparable ideas in lipid nanoparticle formulation and therapeutic targeting. These examples offer insight into how similar challenges have been addressed across different delivery systems.
Did you know? Cancer-targeting innovations like US12144810B1 parallel breakthroughs in lipid-based gene therapy patents, where formulation science plays a decisive role in therapeutic success.
#1. US20110224447A1
This US patent application, US20110224447A1, published in 2011, introduces lipid nanoparticles and novel cationic lipid components for delivering nucleic acids, particularly siRNA, for therapeutic use. It emphasizes precise formulation strategies, diverse administration routes, and analytical validation of RNAi delivery systems.
Below, we have added snapshots from the GPS tool highlighting the relevant snippets from the specification for the similar patents.

What This Patent Introduces To The Landscape
- Cationic lipid-based delivery system – Describes novel cationic lipids within lipid nanoparticles (LNPs) used to encapsulate and deliver siRNA.
- Precision formulation for siRNA delivery – Details siRNA concentration ranges and buffer conditions for stability and biological efficacy.
- Parenteral administration of lipid compositions – Enables intravenous, intramuscular, and subcutaneous administration for therapeutic use.
- RNAi Delivery Vehicle analysis – LNPs analyzed using Triton X-100 treatment and SAX separation, verifying siRNA content and formulation integrity.
- In vivo efficacy studies – Evaluates multiple stereoisomeric LNP formulations for their performance in mice.
How It Connects To EP4241767B1
- Both patents focus on lipid-based carriers optimized for nucleic acid delivery, especially siRNA.
- Each emphasizes structural lipid formulation to enhance cellular uptake and therapeutic index.
- They share a goal of achieving effective systemic delivery with reduced toxicity profiles.
Why This Matters
This earlier work offers insight into the evolution of RNAi delivery platforms. It demonstrates how precise lipid formulation, delivery optimization, and biological validation have long been at the core of advancing nucleic acid therapeutics, principles that EP4241767B1 continues to refine.
Related Read: US12178816B2 and similar patents show how immediate-release cancer formulations rely on polymer carriers, paralleling lipid-based gene therapy patents where delivery chemistry defines success.
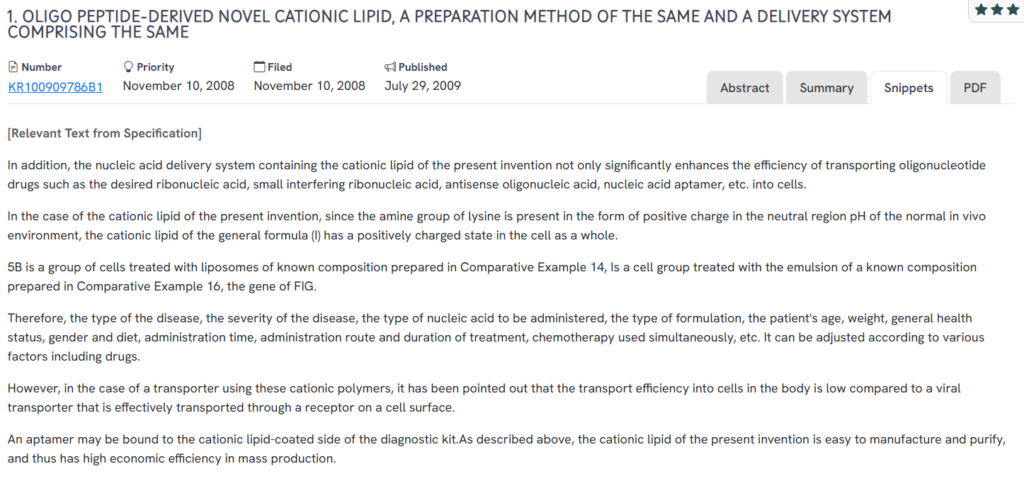
#2. KR100909786B1
This Korean patent, KR100909786B1, published in 2009, presents a novel oligopeptide-derived cationic lipid for nucleic acid delivery. It highlights the efficient intracellular transport of oligonucleotide therapeutics, with a focus on biocompatibility and the potential for large-scale production.
What This Patent Introduces To The Landscape
- Peptide-based cationic lipid design – Features lysine-derived lipids that maintain a positive charge in physiological conditions to aid cell membrane interaction.
- Enhanced transport of therapeutic nucleic acids – Targets delivery of siRNA, antisense oligonucleotides, aptamers, and other RNA-based molecules.
- Customizable administration based on clinical variables – Allows formulation to be tailored to disease type, patient health, and concurrent treatments.
- Low cytotoxicity with efficient purification – Demonstrates cell viability post-treatment and supports scalable manufacturing.
- Diagnostic integration – Includes aptamer-bound lipid surfaces for potential use in targeted diagnostic kits.
How It Connects To EP4241767B1
- Both emphasize improved intracellular delivery of siRNA using custom cationic lipid formulations.
- Each discloses reduced cytotoxicity and increased delivery efficiency in comparison to traditional polymer-based systems.
- Both aim to enable flexible, systemic administration options adaptable to patient-specific therapeutic needs.
Why This Matters
KR100909786B1 contributes to the growing body of work focusing on safe and efficient lipid-based nucleic acid delivery. Its use of peptide-derived cationic structures supports the same therapeutic goals as EP4241767B1: highly targeted delivery, enhanced safety, and readiness for real-world application.
If you want to know more about medical formulations, explore our patent analysis of US6630507B1, which discusses the Neuroprotective Benefits of Cannabinoids.
#3. US20120021044A1
This US patent application, US20120021044A1, published in 2012, discloses a novel cationic lipid and a delivery system designed to enhance the intracellular delivery of nucleic acids and anionic proteins. The invention highlights reduced cytotoxicity and a simple formulation process, both of which are aimed at therapeutic use.

What This Patent Introduces To The Landscape
- Broad-spectrum nucleic acid delivery – Supports intracellular transport of siRNA, DNA, antisense oligonucleotides, and nucleic acid aptamers.
- Delivery of anionic proteins – Enables complex formation through electrostatic interaction between the cationic lipids and negatively charged proteins.
- Reduced cytotoxicity – Offers an alternative to high-toxicity lipids like DOTMA by improving safety without sacrificing gene transfer efficiency.
- Cell fusion and endocytosis mechanisms – Describes liposomal uptake into cells via membrane fusion or endocytosis for therapeutic delivery.
- Simple preparation process – Utilizes amide bond formation between fatty acid derivatives for straightforward synthesis and scale-up.
How It Connects To EP4241767B1
- Both target the effective delivery of therapeutic nucleic acids through engineered cationic lipid systems.
- Each highlights intracellular transport efficiency and low cytotoxicity as design priorities.
- Both explore adaptable lipid compositions to accommodate various nucleic acid drugs and delivery routes.
Why This Matters
US20120021044A1 offers valuable insight into multifunctional delivery platforms that extend beyond siRNA to proteins and aptamers. Its low-toxicity approach and simplified synthesis process align closely with EP4241767B1’s therapeutic goals in safe and efficient systemic delivery.
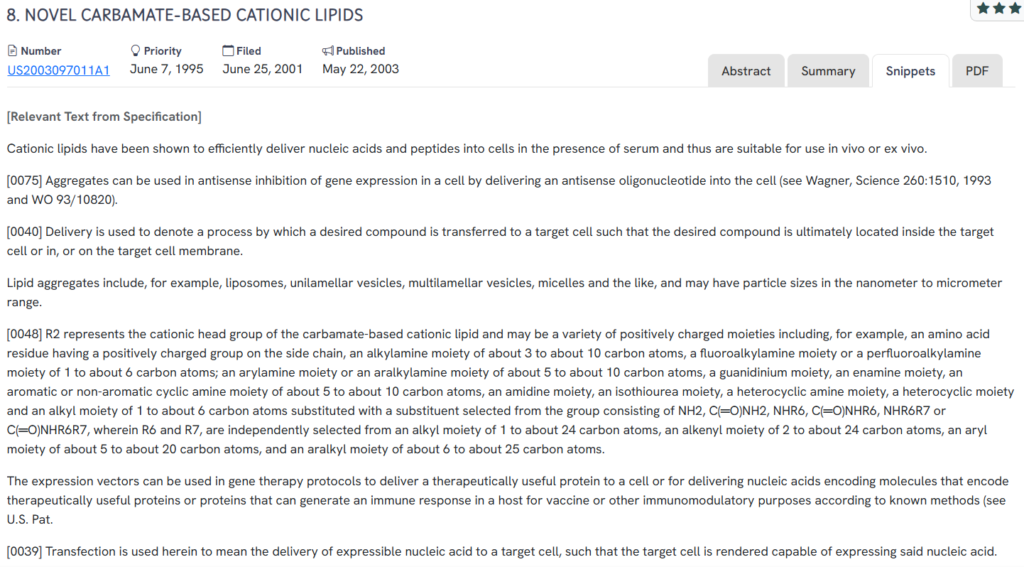
#4. US20030097011A1
This US patent application, US20030097011A1, published in 2003, presents a class of carbamate-based cationic lipids designed for delivering nucleic acids and peptides into target cells. The invention highlights lipid aggregates such as liposomes and vesicles that function under physiological conditions, including in vivo and ex vivo applications.
What This Patent Introduces To The Landscape
- Carbamate-based cationic lipids – Defines cationic lipid structures with a wide range of positively charged head groups to improve compatibility and delivery.
- Versatile lipid aggregate formulations – Includes liposomes, micelles, and unilamellar/multilamellar vesicles for diverse delivery applications.
- Efficient in-serum and in vivo delivery – Demonstrates effective transport of nucleic acids and peptides into cells even in the presence of serum.
- Gene expression and antisense inhibition – Supports delivery of expression vectors and antisense oligonucleotides for gene therapy and immunomodulation.
- Transfection via lipid exchange mechanisms – Emphasizes the importance of lipid chemistry in enabling membrane fusion and cargo delivery.
How It Connects To EP4241767B1
- Both patents aim to improve the delivery of therapeutic nucleic acids through specialized cationic lipid systems.
- Each includes lipid particle design adaptable for gene expression, antisense inhibition, or vaccine delivery.
- They share a focus on using lipid-based delivery under physiological and systemic conditions with minimized toxicity.
Why This Matters
US20030097011A1 provides foundational chemistry for cationic lipids and their role in delivering complex macromolecules. Its early emphasis on lipid structure, particle behavior, and therapeutic gene expression builds a technical bridge to the design philosophy seen in EP4241767B1.
#5. US20050112207A1
This US patent application, US20050112207A1, published in 2005, describes pharmaceutical and diagnostic compositions containing lipid-based nanoparticles designed for targeted delivery to specific tissues and cells. The system is adaptable for carrying therapeutic agents, including oligonucleotides, and supports both treatment and diagnostic applications.

What This Patent Introduces To The Landscape
- Targeted nanoparticle delivery system – Focuses on lipid-based nanoparticles that deliver therapeutic agents directly to diseased tissues or cells.
- Dual therapeutic and diagnostic potential – Supports use for drug delivery as well as disease detection and monitoring.
- Versatility in therapeutic payloads – Includes delivery of oligonucleotides and chemotherapeutic agents such as paclitaxel and etoposide.
- Non-gas-containing nanoparticle formulation – Utilizes stable lipid mixtures for controlled delivery and tissue penetration.
- Internalization validation in biological models – Demonstrates nanoparticle uptake and delivery performance in cellular environments.
How It Connects To EP4241767B1
- Both patents aim to optimize nanoparticle systems for the targeted delivery of nucleic acid-based therapies.
- Each emphasizes improved localization and uptake of therapeutics within cells or tissues.
- Both explore adaptable lipid compositions that support systemic delivery with minimal off-target effects.
Why This Matters
US20050112207A1 extends the utility of lipid nanoparticles beyond RNA delivery to include diagnostics and chemotherapy. Its multifunctional approach aligns with EP4241767B1’s goals of creating flexible, safe, and targeted lipid delivery systems for advanced therapeutic applications.
Note: Delivery defines therapeutic success. While EP3536712B1 focuses on antibodies, lipid carriers highlight complementary design principles for cardiovascular biologics. Read on to know more.
How To Find Related Patents Using Global Patent Search
Understanding the broader patent landscape is key when evaluating drug delivery systems, lipid formulations, or nucleic acid-based therapeutics. This data-backed patent audit is essential for teams working in AI-driven drug discovery, RNA therapeutics, or nanomedicine research.
The Global Patent Search tool streamlines this process, helping users identify technologies that share principles of intracellular targeting, biocompatibility, or therapeutic optimization.
1. Enter the patent number into GPS: Start by entering a patent number like EP4241767B1 into the GPS tool. The platform transforms it into a smart query, which you can refine using terms like cationic lipids, RNA encapsulation, or lipid nanoparticle delivery.

2. Explore conceptual snippets: Instead of comparing features claim-by-claim, GPS now offers curated text snippets. These highlight how other systems encapsulate nucleic acids, enhance uptake, or reduce cytotoxicity.
3. Identify related inventions: The tool reveals patents that address efficient gene transfer, systemic tolerability, or lipid-based drug carriers, showing how similar therapeutic challenges have been tackled.
4. Compare systems, not legal claims: Rather than focusing on formal legal language, GPS compares delivery strategies and formulation mechanisms. This helps users trace functional parallels without having to navigate through legal jargon.
5. Accelerate cross-domain insights: Whether you’re working in RNA therapeutics, nanomedicine, or gene therapy delivery, GPS uncovers related concepts across disciplines that might otherwise go unnoticed.
With this approach, Global Patent Search gives researchers, formulators, and IP teams a concept-driven way to explore overlapping technologies and build more informed strategies. If you’re exploring broader implications of AI in healthcare delivery or drug formulation, don’t miss our overview of AI’s role in healthcare innovation.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.



